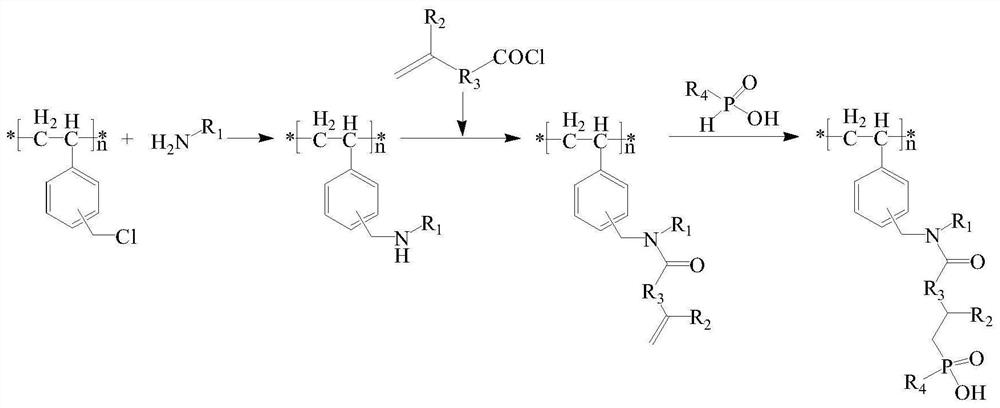
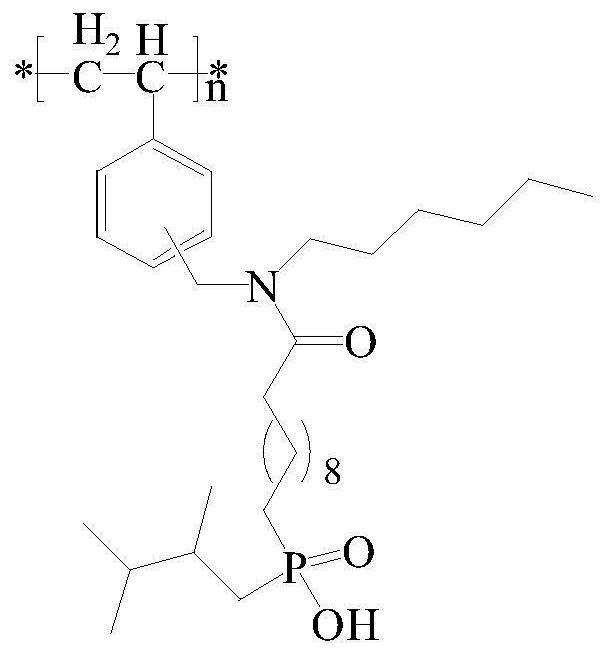
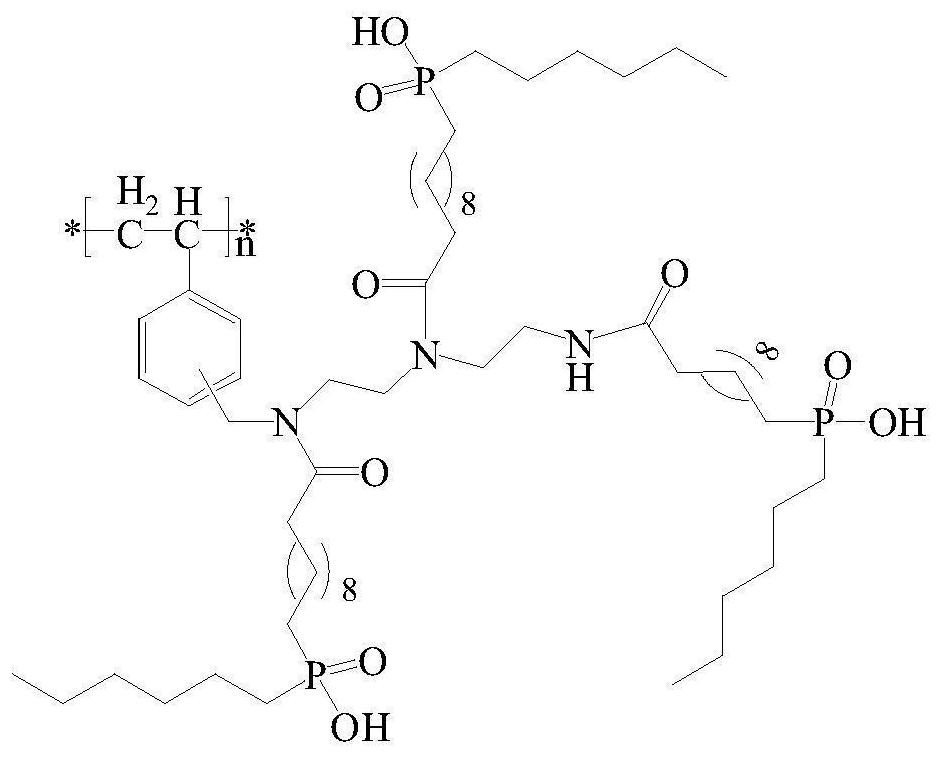
A kind of chlorine sphere modification method of modifying dialkylphosphinic acid functional group
A technology of dialkylphosphinic acid and monoalkylphosphinic acid, which is applied in the modification of chlorine balls, can solve the loss of extraction agent, affect the stability and cycle performance of extraction resin, and prevent To solve problems such as adsorption, achieve good stability, broad prospects for industrial application, and high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Amino-modified chlorine spheres: Weigh 10.2g of dry chlorine spheres, add 150mL of toluene to swell for 18h, add 12.9g of n-hexylamine and 10.6g of triethylamine, and react at 80°C for 18h. Cool down to room temperature, filter, wash with ethanol twice, wash with water until the filtrate is neutral, wash once more with ethanol, and dry in vacuum to obtain amino-modified chlorine spheres.
[0030] (2) Preparation of acid chlorides containing C=C double bonds: add 15.2 g of thionyl chloride to 18.1 g of undecylenic acid, react at 80° C. for 2 h, cool down to room temperature, and remove excess thionyl chloride by rotary evaporator , Add 40mL of anhydrous diethyl ether to obtain a diethyl ether solution of undecylenoyl chloride.
[0031] (3) Chlorine ball grafting C=C double bond: Take 12.0g of amino-modified chlorine balls, add 50mL of anhydrous ether and 8.2g of triethylamine, slowly add the ether solution of undecanoyl chloride through a constant pressure funnel at ...
Embodiment 2
[0034] (1) Amino-modified chlorine spheres: Weigh 20.8g dry chlorine spheres, add 200mL 1,4-dioxane to swell for 12h, add 18.2g diethylenetriamine and 17.6g anhydrous sodium carbonate, and reflux for 12h. Cool down to room temperature, filter, wash with ethanol twice, wash with water until the filtrate is neutral, wash once more with ethanol, and dry at room temperature to obtain amino-modified chlorine spheres.
[0035](2) Chlorine spheres grafting C=C double bonds: Take 25.0g of amino-modified chlorine spheres, add 50mL of tetrahydrofuran and 20.2g of saturated sodium bicarbonate solution, and slowly add 42.2g of undecanoyl chloride through a constant pressure funnel at room temperature. The tetrahydrofuran solution, after the dropwise addition, reacted for 6h. Filter, wash with deionized water until the filtrate is neutral, wash with ethanol, and dry at room temperature.
[0036] (3) Reaction of chlorine balls grafted with C=C double bonds and monohexylphosphinic acid: tak...
Embodiment 3
[0038] (1) Amino-modified chlorine spheres: Weigh 5.2g dry chlorine spheres, add 80mL N,N-dimethylformamide to swell for 10h, add 8.4g 1,6-hexamethylenediamine and 6.6g sodium hydroxide, and reflux Reaction 8h. Cool down to room temperature, filter, wash with ethanol three times, wash with water until the filtrate is neutral, wash with ethanol once more, and dry at room temperature to obtain amino-modified chlorine spheres.
[0039] (2) Preparation of acid chlorides containing C=C double bonds: add 9.2 g of thionyl chloride to 8.1 g of 5-hexenoic acid, react at 80° C. for 2.5 h, drop to room temperature, and remove excess chloride by rotary evaporator sulfoxide, and 20 mL of dry 1,4-dioxane was added to obtain a 1,4-dioxane solution of 5-hexenoyl chloride.
[0040] (3) chlorine ball grafting C=C double bond: get 5.6g amino-modified chlorine balls, add 20mL cyclohexane and 6.2g potassium carbonate solution, slowly add 5-hexenoyl chloride through a constant pressure funnel at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com