Lactoferrin modified patchouli alcohol liposome as well as preparation method and application thereof
A technology of lactoferrin and patchouli alcohol, applied in the field of medicine, can solve the problems of difficult to exert curative effect, poor drug absorption, low bioavailability, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Embodiment 1: the preparation of the liposome of lactoferrin modification
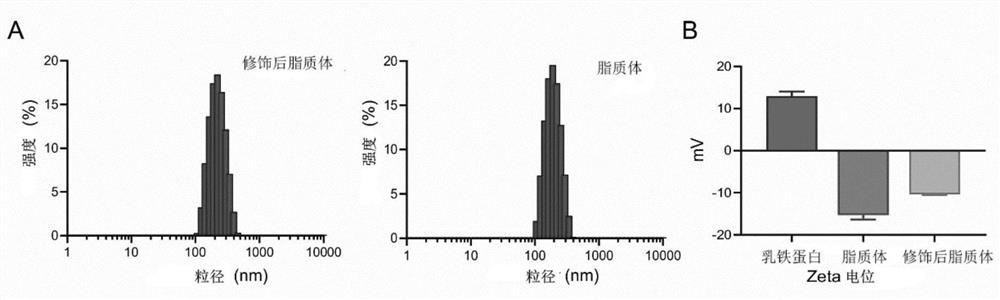
[0063] Weigh egg yolk lecithin, cholesterol, distearoylphosphatidylethanolamine-polyethylene glycol 2000, distearoylphosphatidylethanolamine-N-hydroxysuccinimide-polyethylene glycol 2000 (30:6:6 :1,w / w) (both purchased from Shanghai Aiweite Pharmaceutical Technology Co., Ltd.) and patchouli alcohol (Chengdu Master Biological Technology Co., Ltd.), dissolved in chloroform. The resulting solution was evaporated to dryness with chloroform under reduced pressure at 37°C to form a thin film. Phosphate buffer solution was added to the film for hydration to form lipid water dispersion. Ultrasonic cell disruptor (JY92-IIN, Ningbo Xinzhi Biotechnology Co., Ltd.) was used to sonicate for 5 min at 5% power. Afterwards, use a membrane extruder to repeatedly extrude the liposomes until the liposomes pass through a membrane with a particle size of 200 nm to form liposomes with uniform particle sizes. A Sep...
Embodiment 2
[0064] Example 2: Characterization of lactoferrin-modified liposomes
[0065] (1) Reaction of lactoferrin with N-hydroxysuccinimide ester
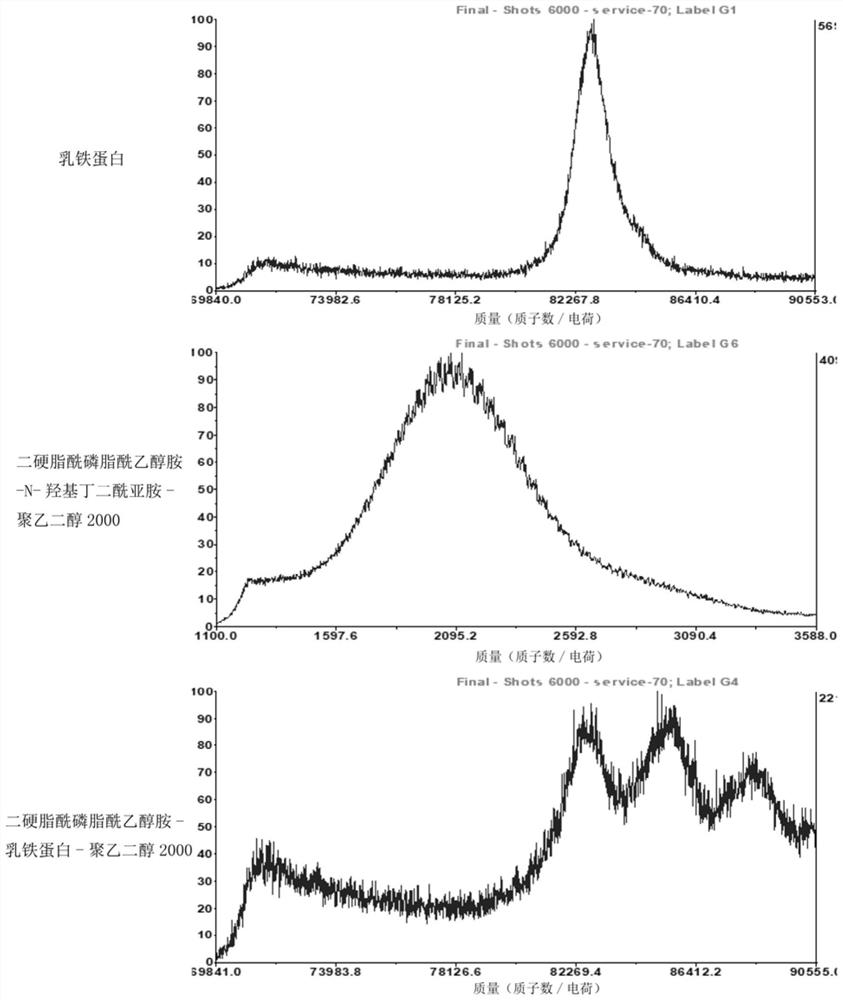
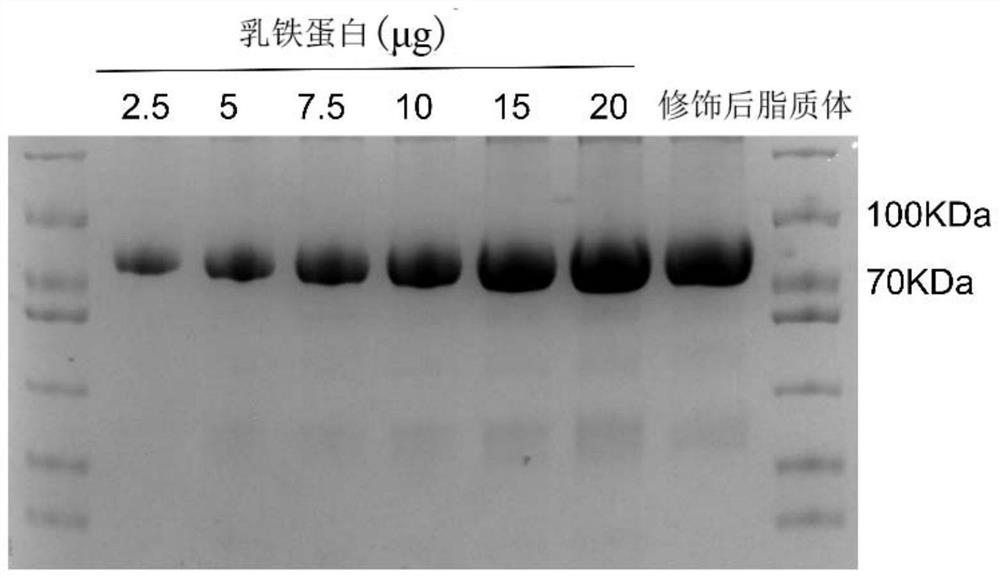
[0066] Weigh 1 mg of lactoferrin, prepare a 1 mg / mL solution with ultrapure water, and use the same method to prepare a distearoylphosphatidylethanolamine-N-hydroxysuccinimide-polyethylene glycol 2000 solution. The lactoferrin solution was mixed with the distearoylphosphatidylethanolamine-N-hydroxysuccinimide-polyethylene glycol 2000 solution, placed on a shaker at 4°C, and incubated for 12-16 hours. Take the reacted solution, place it in a dialysis bag with a molecular weight of 14K, and take it out after dialysis with magnetic stirring for 24 hours. At this time, the reaction product distearoylphosphatidylethanolamine-lactoferrin-polyethylene glycol 2000 is obtained. Mix lactoferrin solution, distearoylphosphatidylethanolamine-lactoferrin-polyethylene glycol 2000 solution with sinapinic acid matrix (Shanghai Yuanye Biotechnology Co., Lt...
Embodiment 3
[0081] Example 3: Cell Safety Evaluation
[0082] Bone marrow-derived macrophages (extracted from Balb / c mice) were mixed at 1×10 per well 4 Cells were seeded in 96-well plates, 100 μL per well, and induced to differentiate into M1 macrophages. After the cell differentiation was completed, they were divided into three groups: free patchouli alcohol, liposomes prepared in Example 1, and lactoferrin-modified liposomes prepared in Example 1, and each group was administered according to a certain concentration gradient. After administration, the culture was continued for 24 hours, and then 20 μL of thiazolium blue (MTT) (Sigma-Aldrich, USA) was added to each well, and the incubation was continued for 4 hours, after which the supernatant was aspirated to leave purple crystals. Add 200 μL dimethyl sulfoxide to each well, place on a shaker at room temperature until the purple crystals are completely dissolved, use a microplate reader (Multiskan, ThermoFisher, USA) to detect the OD v...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com