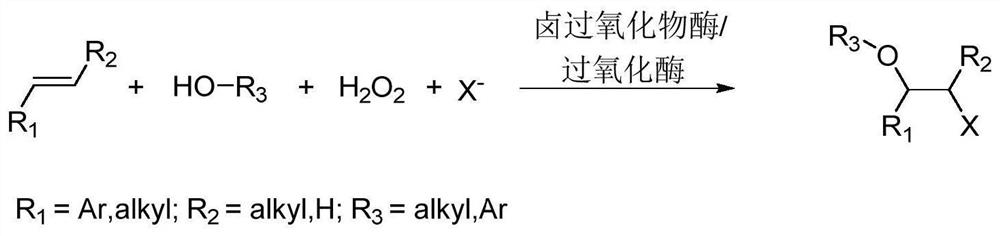Method for preparing beta-halogenated ether and beta-halogenated alcohol under catalysis of peroxidase
A peroxidase and peroxidase technology, which is applied in the biological field to achieve the effects of simple raw materials, mild reaction conditions and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0082] The preparation method of UPO enzyme in the following examples is as follows:
[0083] Pichia pastoris cell culture broth containing rAaeUPO was clarified by centrifugation at 8000 rpm for 2 hours at 4°C. The supernatant was filtered through a 20 μm filter and kept at -80°C. rAaeUPO activity was determined using the ABTS assay in NaPi buffer at pH 5.0;
[0084] Protein Purification: The supernatant was concentrated and dialyzed against 100 mM sodium phosphate, pH 7. AaeUPO was purified in one step using NGC chromatography system (Biorad). Separation was performed on a Q Sepharose FF 30-mL cartridge at a flow rate of 5 mL / min. After 90 mL, the retained protein was eluted with a gradient of 0-50% NaCl in 450 mL, followed by a gradient of 50-100% in 50 mL and 100% NaCl in 75 mL of peroxidase activity followed by an H 2 o 2 Oxidation in the presence of ABTS, appropriate fractions were pooled, concentrated and dialyzed against 100 mM sodium phosphate buffer (pH 7). Pur...
Embodiment 1
[0085] Embodiment 1, the preparation of compound 1-bromo-2-ethoxycyclohexane:
[0086]
[0087] In a 100mL reaction flask, add 31.5mL of pH=6 phosphate buffer solution (sodium dihydrogen phosphate 100 mM), add absolute ethanol (13.5mL) to the above buffer solution, and then sequentially add 0.93mL with a mass fraction of 30 % hydrogen peroxide solution (concentration is 5.38mol / L, concentration in the reaction system is 100mM), 0.59g potassium bromide and 0.820g cyclohexene, finally add 150μL VCPO enzyme (concentration of raw material enzyme is 13.5mg / mL, reaction The enzyme concentration in the system is 500nM). After reacting for about 3 hours, 0.59g potassium bromide, 0.93mL hydrogen peroxide (concentration: 5.38mol / L, concentration in the reaction system: 100mM) and 0.820g cyclohexene were added for a total of three times. Add in separate batches to ensure maximum catalytic activity of the enzyme.
[0088] The above reaction system was stirred and reacted at 30°C for ...
Embodiment 2
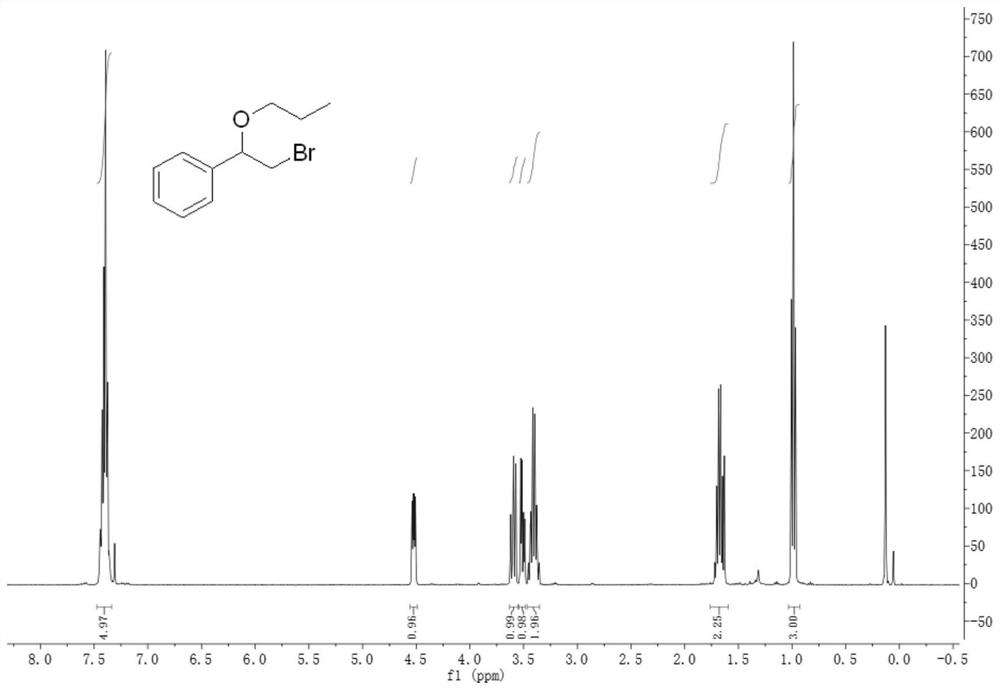
[0089] Embodiment 2, the preparation of compound (2-bromo-1-propoxyethyl) benzene:
[0090]
[0091]In a 100mL reaction flask, add 31.5mL of phosphate buffer solution (same pH=6 as above), then add 13.5mL of absolute ethanol to dissolve in the buffer solution, and then add 0.93mL of 30% hydrogen peroxide solution in sequence (Concentration is 5.38mol / L, the concentration in the reaction system is 100mM), 0.59g potassium bromide and 0.16g styrene, finally add 150μL VCPO enzyme (the same as the above enzyme concentration is 13.5mg / mL, the enzyme concentration in the reaction system is 500nM). After reacting for about 3 hours, 0.59 g of potassium bromide, 0.93 mL of hydrogen peroxide (concentration: 5.38 mol / L) and 0.16 g of styrene were added three times in total.
[0092] The above reaction system was stirred and reacted in a water bath at 30°C for 9 hours, then the reaction was terminated, and 100 mL of ethyl acetate was added for extraction twice in total. The organic pha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com