Antibacterial oligopeptide LBD-S and application and medicine thereof
A LBD-S, short peptide technology, applied in the direction of antibacterial drugs, peptides, depsipeptides, etc., can solve the problems of eukaryotic cytotoxicity and the unsatisfactory activity of natural antibacterial peptides, and achieve high antibacterial activity, low hydrophobicity, Hydrophilic and lipophilic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
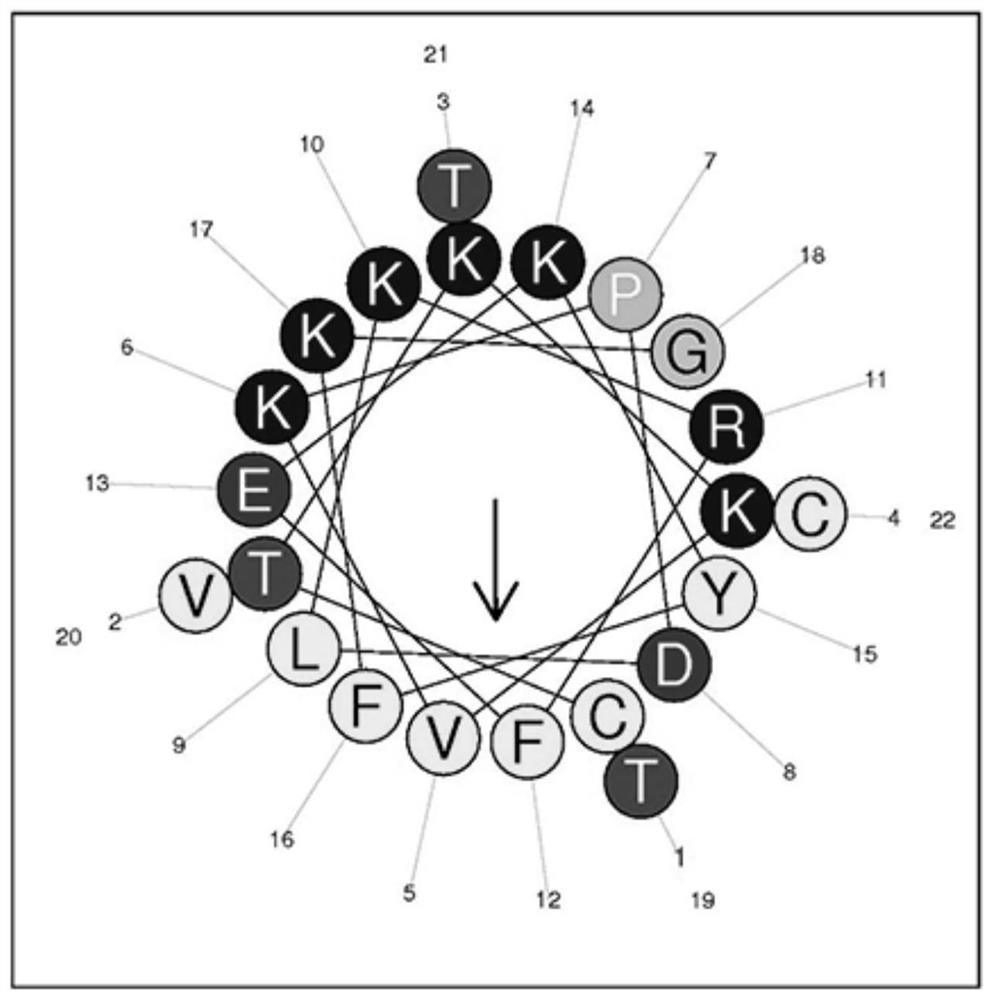
[0046] Example 1 Transformation of antimicrobial peptide LBD short peptide based on MjALF-D
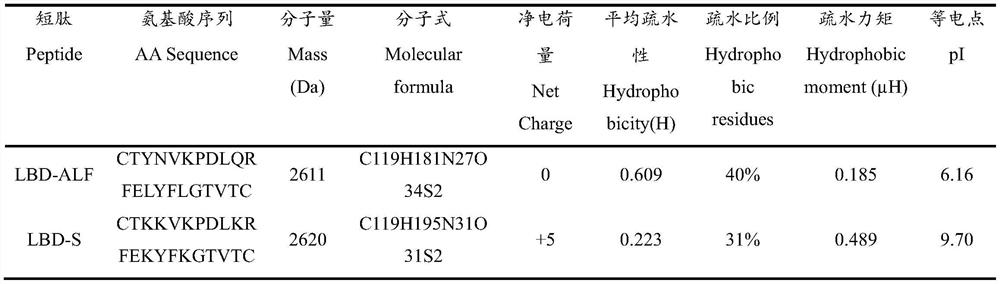
[0047]According to the analysis of the physical and chemical properties and structural information of the LBD short peptide of MjALF-D, the analysis software uses the ProtParam online analysis software (http: / / www.expasy.org / tools / protparam) in the Expert Protein Analysis Software (ExPASy) website And The Antimicrobial Peptide Database (APD, http: / / aps.unmc.edu / AP / main.php) online analysis software to obtain relevant parameters of the peptide. The analysis of the arrangement of hydrophilic and hydrophobic amino acids of short peptides and the helical wheel projection were performed using the online analysis software Heliquest analysis (http: / / heliquest.ipmc.cnrs.fr / cgi-bin / ComputParams.py) (Gautier et al., 2008 )conduct. Sequence alignment of the LBD short peptide (LBD-ALF) of MjALF-D in the APD antimicrobial peptide system, and amino acid substitutions were carried out according to ...
Embodiment 2
[0054] The artificial synthesis of embodiment 2 antibacterial short peptide LBD-S short peptide
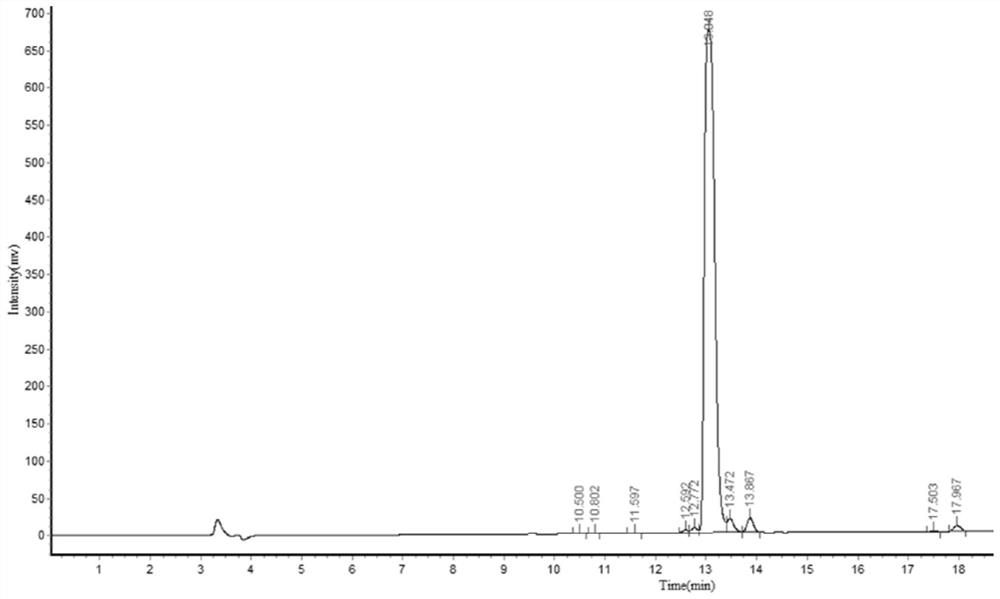
[0055] The antibacterial LBD short peptide that has been modified and designed is synthesized. The synthesis method is based on solid-phase chemical synthesis. The synthesis direction is from the C-terminal to the N-terminal of the LBD-S short peptide, and the N-terminal is acetylated, and the C-terminal is amidated to enhance the stability of the synthetic peptide. Sexuality. The carboxy-terminus and amino-terminus of LBD-S are cysteine, forming a disulfide bond. The synthesized LBD-S short peptide was purified by reverse-phase high-performance liquid chromatography and its purity was tested. The results are as follows: figure 2 shown.
[0056] from figure 2 It can be seen from the figure that the synthesized antibacterial short peptide LBD-S has only one prominent peak, and the purity is high (95.0749%).
Embodiment 3
[0057] The MIC of embodiment 3 antimicrobial short peptide LBD-S and MBC measure
[0058] The detection and analysis of protein minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) is carried out by liquid growth inhibition assay, and the specific determination method refers to the following literature:
[0059] [1] Ying P, Libing Z, Mao Y, et al.The antibacterial activity and mechanism analysis of piscidin 5 like from Larimichthys crocea[J].2019,92:43-49.
[0060] The results are shown in Table 2.
[0061] Table 2 MIC and MBC of LBD-ALF and modified LBD-S short peptide (unit: μM)
[0062]
[0063]
[0064] Note: NT: not detected.
[0065] As can be seen from Table 2, the modified antibacterial short peptide LBD-S is effective against Gram-positive bacteria (including Bacillus subtilis, Corynebacterium glutamicum, Micrococcus lyticus and Micrococcus luteus) and Gram-positive bacteria. Negative bacteria (including Photobacterium mermaidus...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com