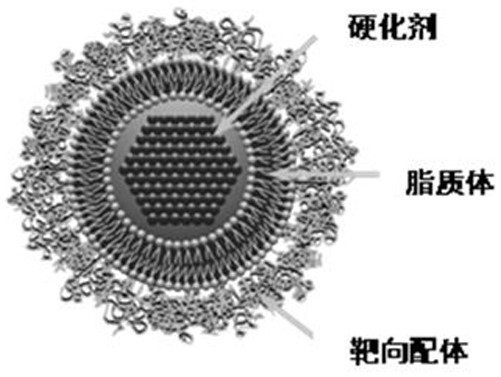
Targeted liposome drug delivery system and preparation method and application thereof
A technology targeting liposomes and delivery systems, applied in liposome delivery, drug delivery, drug combination, etc., can solve the problems of low drug efficacy, high toxicity and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] S1: Weigh 30 mg of HA (M=8000), swell it in 10 mL distilled water, dissolve it ultrasonically, add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) 60 mg and N-hydroxysulfosuccinimide (NHS) 90 mg, add sodium hydroxide solution to adjust the pH value to 7.5, activate in a water bath at 37°C for 24 h, add dropwise 72 mg DSPE-PEG-NH 2 The suspension was reacted overnight, and the excess reactants and by-products in the reaction solution were removed by dialysis using a dialysis membrane with a pore size of 5000 to prepare DSPE-PEG-HA;
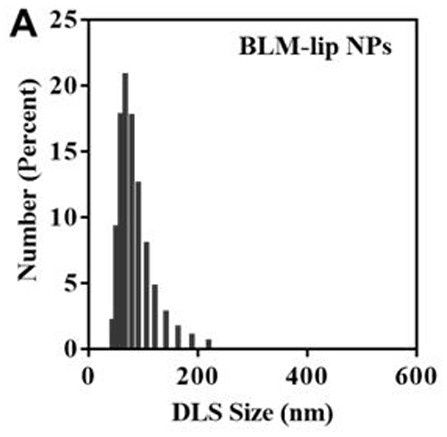
[0040]S2: Weigh 120 mg of lecithin (Spc), 30 mg of cholesterol (Cho), and 6 mg of bleomycin into a single-necked bottle, add 10 mL of chloroform solvent, ultrasonically dissolve it fully, and place the solvent in a rotary evaporator. Evaporate, add 3 mL (pH=7.4) of PBS buffer solution and 2 ml of DSPE-PEG-HA in PBS solution (2 mg / mL), hydrate in a water bath at 60 °C for 1 h, and ultrasonicate the suspension with a probe; ...
Embodiment 2
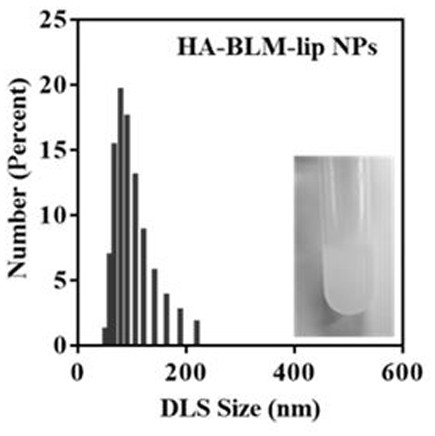
[0042] The preparation materials and method are the same as those in Example 1, the difference is only in the liposome extrusion process, specifically, the suspension after ultrasonic treatment of the probe is sequentially extruded 20 times through 450nm and 200nm polycarbonate membranes respectively to obtain HA- BLM-lip NPs.
Embodiment 3
[0044] The preparation materials and method are the same as in Example 1, the difference is only in the liposome extrusion process, specifically, the suspension after the ultrasonic treatment of the probe is sequentially extruded 20 times through the polycarbonate membranes of 450nm, 200nm and 100nm respectively to obtain HA-BLM-lipNPs.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com