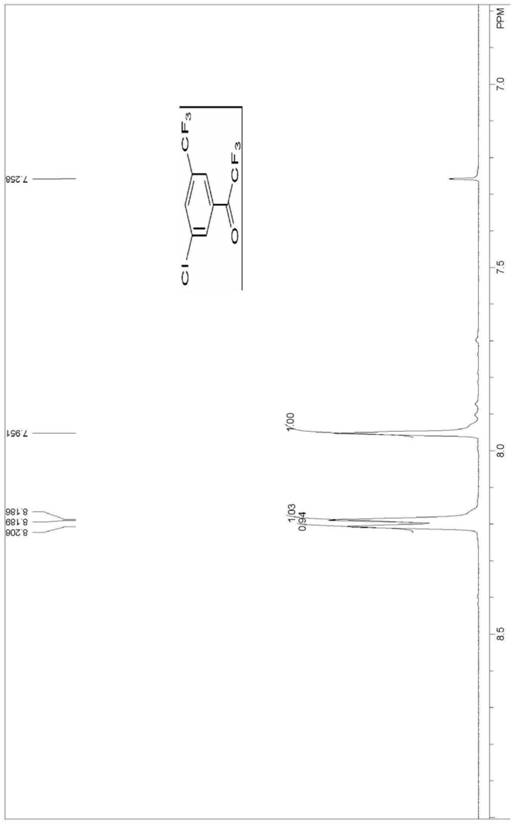
Method for synthesizing 3-chloro-5-trifluoromethyl trifluoroacetophenone
A technology of trifluoromethyl trifluoroacetophenone and trifluoromethyl aniline, which is applied in the field of synthesizing 3-chloro-5-trifluoromethyl trifluoroacetophenone, can solve problems that have not been reported, and achieve Save production time and cost, easy to adjust the reaction conditions, simplify the post-processing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
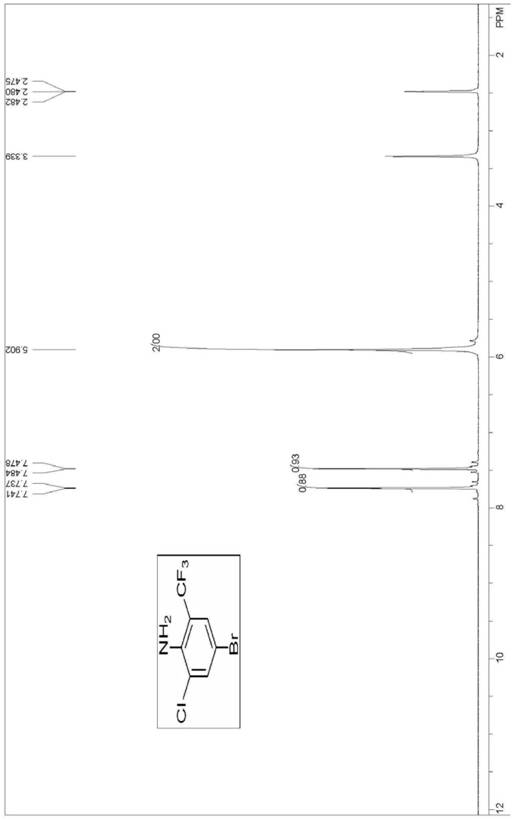
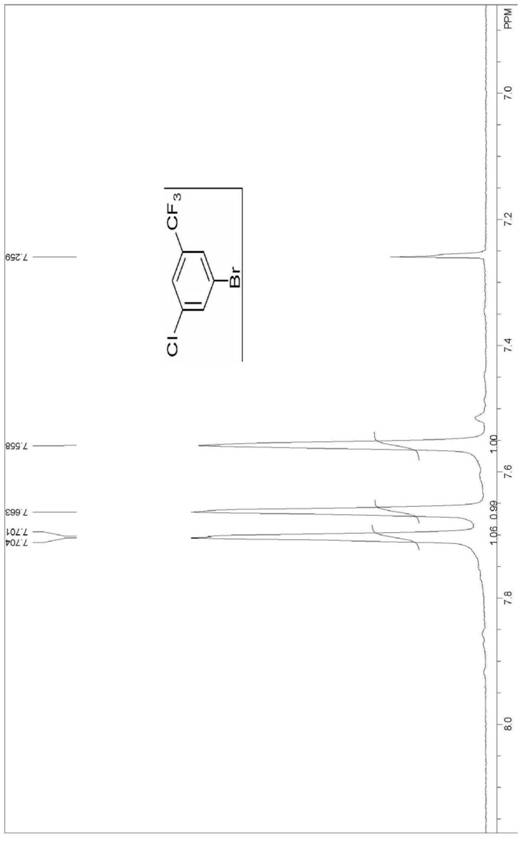
[0074] (1) Synthesis of 4-bromo-2-chloro-6-trifluoromethylaniline
[0075] Add 200g of o-aminobenzotrifluoride (1eq), 1kg of dichloromethane and 0.1g of iron powder to a 2L three-necked flask with an exhaust gas absorbing device in sequence; start stirring, cool down to 0°C, and drop 144.7g into the three-necked flask Cold (about -15°C) bromine monochloride (1.01eq), control the internal temperature at 0-5°C, after 1-3h dropwise addition, then keep warm for 2h; then, naturally heat up to 25°C, keep warm for bromination reaction , gas phase monitoring of the reaction process of the raw material o-aminobenzotrifluoride in the bromination reaction, monitoring shows: the bromination reaction is carried out for 2h, and the reaction of the raw material o-aminobenzotrifluoride is completed.
[0076] After the completion of the o-aminobenzotrifluorotoluene reaction, add 169.2 g of sulfonyl chloride (1.01 eq) dropwise to the reaction solution at 25° C., control the internal temperature...
Embodiment 2
[0090] (1) Synthesis of 4-bromo-2-chloro-6-trifluoromethylaniline
[0091] Add 120 g of o-aminobenzobenzotrifluoride (1 eq), 800 g of acetonitrile and 0.1 g of aluminum trichloride to a 2 L three-necked flask in sequence, and start stirring.
[0092] Cool down to 0°C, add 117.1g of dibromohydantoin solid (0.55eq) in batches (3g each time, 2h for feeding), control the internal temperature at 0-5°C, keep warm for 4h after addition; then naturally raise the temperature to 25°C , heat preservation for bromination reaction, gas phase monitoring of the reaction process of the raw material o-aminobenzotrifluoride in the bromination reaction, monitoring shows: the bromination reaction is carried out for 5h, and the reaction of the raw material o-aminobenzotrifluoride is completed.
[0093] After the reaction of o-aminotrifluorotoluene is completed, continue to add 64g of TCCA solid (0.37eq) to the reaction solution in batches at 25°C (1g each time, 3h for feeding), control the interna...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com