Photosensitive COFs catalyst and method for catalytic synthesis of thiophosphate derivatives
A technology for synthesizing thiophosphoric acid and thiol derivatives, applied in chemical instruments and methods, catalysts for physical/chemical processes, compounds of elements of Group 5/15 of the periodic table, etc. Affecting industrial applications and other issues, achieving the effect of strong industrial application prospects, wide substrate applicability, and fewer by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
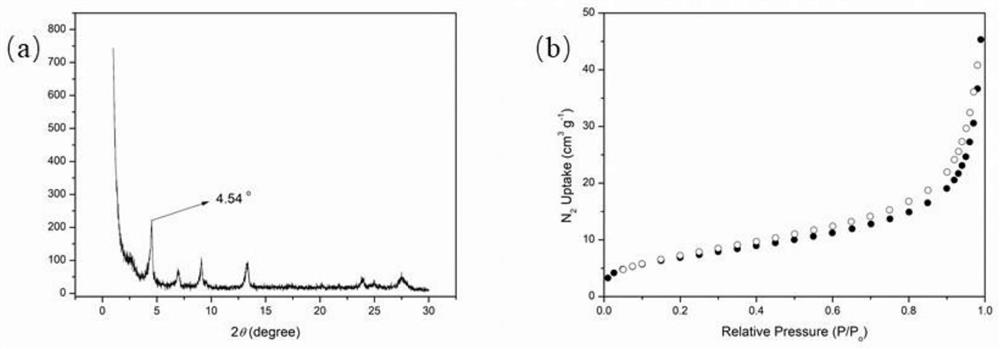
[0029] (1) Synthesis of photosensitive COF-1 catalyst
[0030]Synthesis of photosensitive COFs-1: 1,3,6,8-tetrakis-(4-formylphenyl)-pyrene (0.08mmol, 49.5mg), 1,1'-[1,4-phenylenebis (Methylene)]bis(1-pyridinium) dichloride (0.16mmol, 53.1mg), 6M potassium tert-butoxide aqueous solution (150uL) and o-dichlorobenzene / n-butanol (volume ratio 2:1) were mixed Add 1.5mL of solvent to a 10mL ampoule. Immerse the ampoule in liquid nitrogen to freeze for 5 minutes, connect the vacuum pump to pump air for 5 minutes under freezing conditions, stop pumping, and place the ampoule at room temperature until the liquid in the bottle melts. The above-mentioned "freezing-pumping-thawing" operation was performed twice again. The ampoule is frozen, and the neck of the ampoule is heated and sealed under pumping. The sealed ampoule was placed at room temperature to completely melt the liquid, and the ampoule was placed in an oven previously heated to 150° C., and left to react for 72 hours. Aft...
Embodiment 2
[0038] The photosensitive COF-1 catalyst was synthesized as in Example 1.
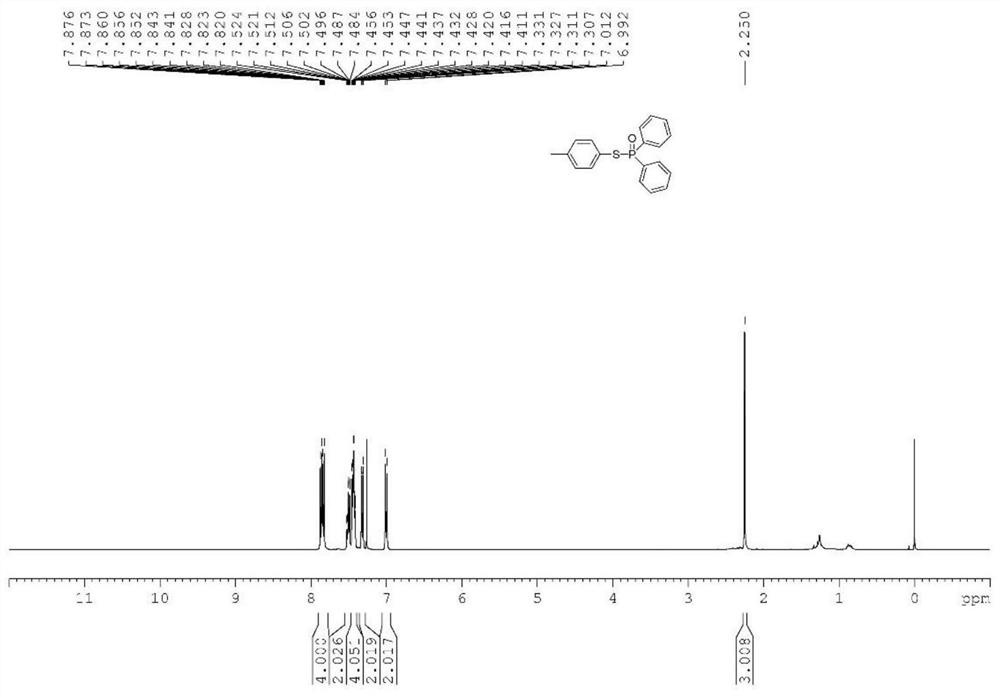
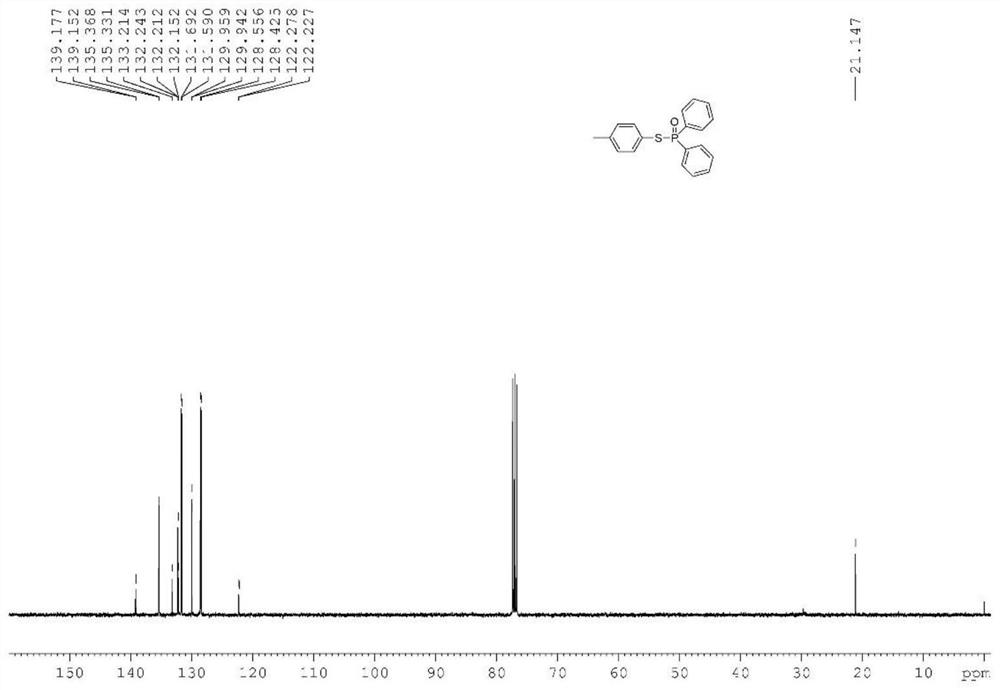
[0039] Synthesis of target compound 2: 202 mg of diphenylphosphine oxide, 320 mg of 2-thionaphthol, 5 mg of photosensitive COF-1 catalyst, and 5 g of DMF were weighed in a 100 ml flask. A stir bar was added thereto, and then stirred for 6 h at 25° C. under an air atmosphere under ultraviolet light irradiation. After the reaction was finished, the catalyst was removed by filtration, the filtrate solvent was removed by a rotary evaporator, and then 2292 mg of phosphorothioate derivatives were obtained by column chromatography separation, with a yield of 81%.
[0040]
[0041] NMR data of target compound 2: 1 H NMR (600MHz, CDCl 3 )δ7.99(s,1H),7.91-7.85(m,4H),7.75-7.67(m,3H),7.52-7.41(m,9H); 13 C NMR (150MHz, CDCl 3 )δ135.3(d, J=5.0Hz), 133.5(d, J=1.6Hz), 132.9(d, J=1.2Hz), 132.5(d, J=107.0Hz), 132.3(d, J=3.0 Hz), 131.6(d, J=10.3Hz), 131.5(d, J=3.3Hz), 128.6(d, J=1.1Hz), 128.5(d, J=13.2Hz), 127.8,...
Embodiment 3
[0044] The photosensitive COF-1 catalyst was synthesized as in Example 1.
[0045] Synthesis of target compound 3:
[0046] In a 100ml flask, 708mg of bis(4-phenylphenyl)phosphine oxide, 500mg of p-methylthiophenol, 8mg of photosensitive COF-1 catalyst, 7g of acetonitrile, and 7g of water were weighed. A stirrer bar was added thereto, and then stirred for 12 h at 25° C. in an air atmosphere under the irradiation of a blue LED light. After the reaction was completed, the catalyst was removed by filtration, the filtrate solvent was removed by a rotary evaporator, and then 743 mg of phosphorothioate derivative 3 was obtained by column chromatography separation, with a yield of 78%.
[0047]
[0048] NMR data of target compound 3: 1 H NMR (400MHz, CDCl 3 )δ7.98-7.92(m,4H),7.70-7.67(m,4H),7.62-7.60(m,4H),7.49-7.45(m,4H),7.42-7.38(m,4H),7.04( d,J=8.1Hz,2H),2.27(s,3H); 13 C NMR (100MHz, CDCl 3 )δ145.0(d, J=3.0Hz), 139.8, 139.2(d, J=2.3Hz), 135.4(d, J=3.7Hz), 132.2(d, J=10.6H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com