Two-dimensional covalent organic framework materials based on phenoxazine and their preparation methods and applications
A technology of covalent organic framework and phenoxazine, which is applied in secondary batteries, final product manufacturing, electrolyte storage battery manufacturing, etc., can solve problems such as slow redox kinetics, low active site utilization, poor cycle stability, etc. , to achieve fast redox kinetics, good application prospects, and high crystallinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Another aspect of the embodiments of the present invention also provides a method for preparing a phenoxazine-based two-dimensional covalent organic framework material, comprising:
[0037] Under a protective atmosphere, a uniformly mixed reaction system containing 10-methylphenoxazine-2,7-diamine, aryl aldehydes, aqueous acetic acid and solvent was reacted at 100-150 °C for 3-7 days to prepare Two-dimensional covalent organic framework materials based on phenoxazine were obtained.
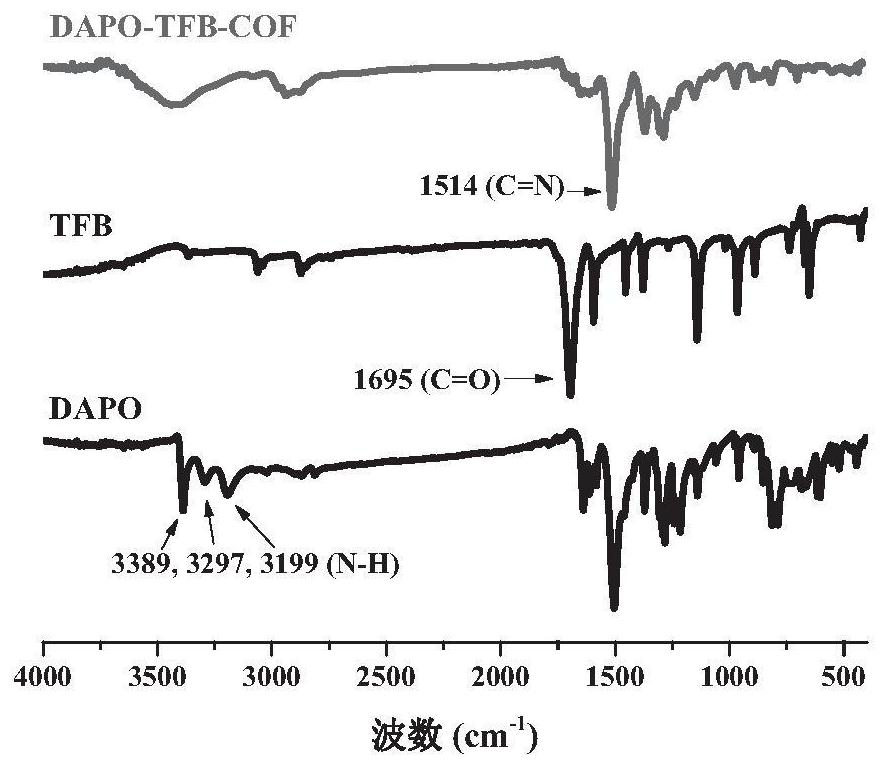
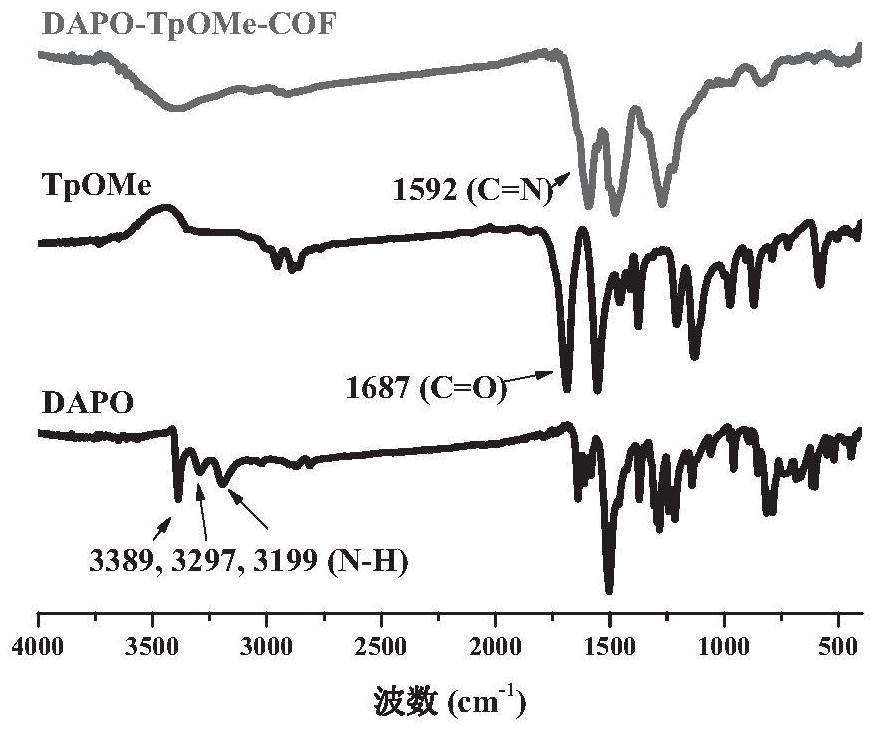
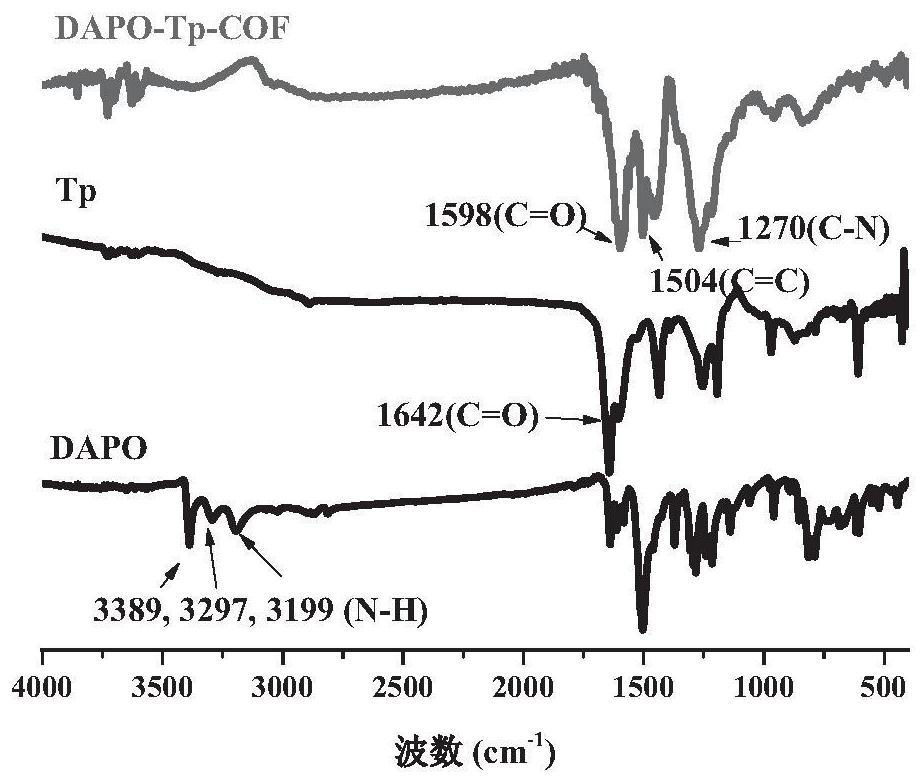
[0038] In some specific embodiments, the aryl aldehydes include 1,3,5-benzenetricarbaldehyde (TFB), 2,4,6-trimethoxybenzene-1,3,5-tricarbaldehyde ( Any one of TpOMe) and 2,4,6-trihydroxybenzene-1,3,5-tricarbaldehyde (Tp).
[0039] Further, the concentration of the acetic acid aqueous solution is 3-9 mol / L.
[0040] Further, the solvent is a high boiling point solvent with a boiling point range of 80-180°C.
[0041] Further, the solvent includes any one or a combination of two or more of ...
Embodiment 1
[0067] Preparation of phenoxazine-based two-dimensional covalent organic framework material DAPO-TFB-COF:
[0068] Add 102.1mg 10-methylphenoxazine-2,7-diamine and 48.6mg 1,3,5-benzenetricarboxaldehyde to a 10mL Slack tube, then add 2mL acetonitrile, and after ultrasonic dissolving, add 0.6mL with a concentration of 3 mol / L acetic acid aqueous solution, then the reaction system was degassed in liquid nitrogen by three cycles of freezing-vacuum-thawing cycles, and the reaction mixture was sealed in a constant temperature oil bath, heated to 120°C, and kept for 3 days. After the reaction was completed, it was cooled to room temperature, and the obtained mixture was centrifuged to collect the solid, then centrifuged and washed with N,N-dimethylformamide and tetrahydrofuran, and vacuum-dried at 80 °C for 12 h to obtain a reddish-brown powder DAPO-TFB-COF. The yield was DAPO-TFB-COF. is 98%.
Embodiment 2
[0070] Preparation of phenoxazine-based two-dimensional covalent organic framework material DAPO-TFB-COF:
[0071] Add 102.1 mg of 10-methylphenoxazine-2,7-diamine and 48.6 mg of 1,3,5-benzenetricarboxaldehyde to a 10 mL Slack tube, then add 2 mL of dioxane, dissolve by ultrasonic and add 0.6 Aqueous acetic acid with a concentration of 6 mol / L in mL. The reaction system was then degassed in liquid nitrogen in three cycles of freeze-vacuum-thaw cycles. The reaction mixture was sealed in a constant temperature oil bath, heated to 120° C. and kept for 3 days. After the reaction was completed, it was cooled to room temperature. The obtained mixture was centrifuged to collect solids, and then washed with N,N-dimethylformamide and tetrahydrofuran by centrifugation. Vacuum drying at 80°C for 12h gave DAPO-TFB-COF as a reddish-brown powder with a yield of 98%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com