Heterocycle-containing dehydroabietylformamide compound as well as preparation method and application thereof
A technology for abietylformamide and dehydroabietylamine, which is applied in the field of dehydroabietylformamide compounds and their preparation, can solve problems such as no dehydroabietylformamide compounds, and achieves novel molecular structure and reaction conditions. Easy-to-control, easy-to-prepare effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
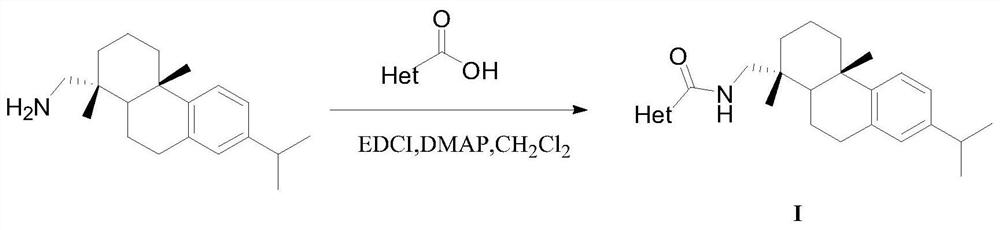
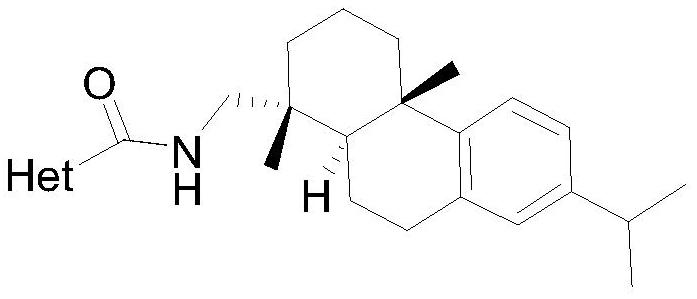
[0015] The preparation of the dehydroabietyl carboxamide compound containing heterocycle:
[0016] Take 2-methylfuran-3-carboxylic acid (1.20mmol) and dehydroabietylamine (1.0mmol) in a one-necked bottle, add dichloromethane (10mL) to dissolve, add catalyst 4-dimethylaminopyridine (0.20mL) under stirring mmol) and condensing agent EDCI (1.20mmol), after addition, react at 25°C for 6-10h, TLC detection, after the reaction is complete, wash the organic layer with saturated sodium bicarbonate solution, wash with water, wash with saturated saline, and dry over anhydrous sodium sulfate , filtered with suction, concentrated under reduced pressure to remove dichloromethane to obtain a crude product, purified by silica gel column chromatography (eluent dichloromethane) and dried to obtain the target compound.
[0017]
[0018] Compound I-1. Yield, 75%, white wax. 1 H NMR (600MHz, CDCl 3 )δ7.22(d, J=2.0Hz, 1H), 7.16(d, J=8.2Hz, 1H), 6.99(dd, J 1 =8.1Hz,J 2 =1.5Hz,1H),6.89(s,1H),...
Embodiment 2
[0020]
[0021] Take 2-fluoropyridine-3-carboxylic acid (1.20mmol) and dehydroabietylamine (1.0mmol) in a one-necked bottle, add dichloromethane (10mL) to dissolve, add the catalyst 4-dimethylaminopyridine (0.20mmol) under stirring and condensing agent EDCI (1.20mmol), after adding, react at 25°C for 6-10h, TLC detection, the reaction is completed, the organic layer is washed with saturated sodium bicarbonate solution, washed with water, washed with saturated saline, dried over anhydrous sodium sulfate, pumped Filtrate, concentrate under reduced pressure to remove dichloromethane to obtain a crude product, purify by silica gel column chromatography (eluent dichloromethane) and dry to obtain the target compound I-2, yield, 75%, white solid; m.p.79-80.5°C.m.p. 79-80.5°C; 1 H NMR (400MHz, CDCl 3 )δ8.49–8.45(m,1H),8.21(d,J=4.5Hz,1H),7.26-7.23(m,1H),7.08(d,J=8.2Hz,1H),6.90(d,J =8.1Hz,1H),6.85–6.79(m,2H),3.39(dd,J 1 =13.6Hz,J 2 =6.1Hz, 1H), 3.29(dd, J1=13.6Hz, J2=6.3Hz, 1H), ...
Embodiment 3
[0023]
[0024] Take 2-chloropyridine-3-carboxylic acid (1.20mmol) and dehydroabietylamine (1.0mmol) in a single-necked bottle, add dichloromethane (10mL) to dissolve, add the catalyst 4-dimethylaminopyridine (0.20mmol) under stirring and condensing agent EDCI (1.20mmol), after adding, react at 25°C for 6-10h, TLC detection, the reaction is completed, the organic layer is washed with saturated sodium bicarbonate solution, washed with water, washed with saturated saline, dried over anhydrous sodium sulfate, pumped Filter, concentrate under reduced pressure to remove dichloromethane to obtain a crude product, purify by silica gel column chromatography (eluent dichloromethane) and dry to obtain the target compound I-3. Yield 80%, white solid; m.p.86-87°C. 1 H NMR (400MHz, CDCl 3 )δ8.29(d, J=4.3Hz, 1H), 7.93(d, J=7.4Hz, 1H), 7.18–7.16(m, 1H), 7.06(d, J=8.1Hz, 1H), 6.89( d,J=8.1Hz,1H),6.79(s,1H),6.58(t,J=5.4Hz,1H),3.30(d,J=9.4Hz,2H),2.87–2.68(m,3H), 2.21(d, J=12.8Hz, 1H), 1.88...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com