Beta-1, 4 type glucuronic acid/mannuronic acidstraight-chain hybrid oligosaccharide as well as preparation method and application thereof
A technology of mannuronic acid and glucuronic acid, which is applied in the field of pharmaceutical compounds, can solve problems such as difficult quality control, and achieve the effects of simple quality control, simple preparation method, and easy industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
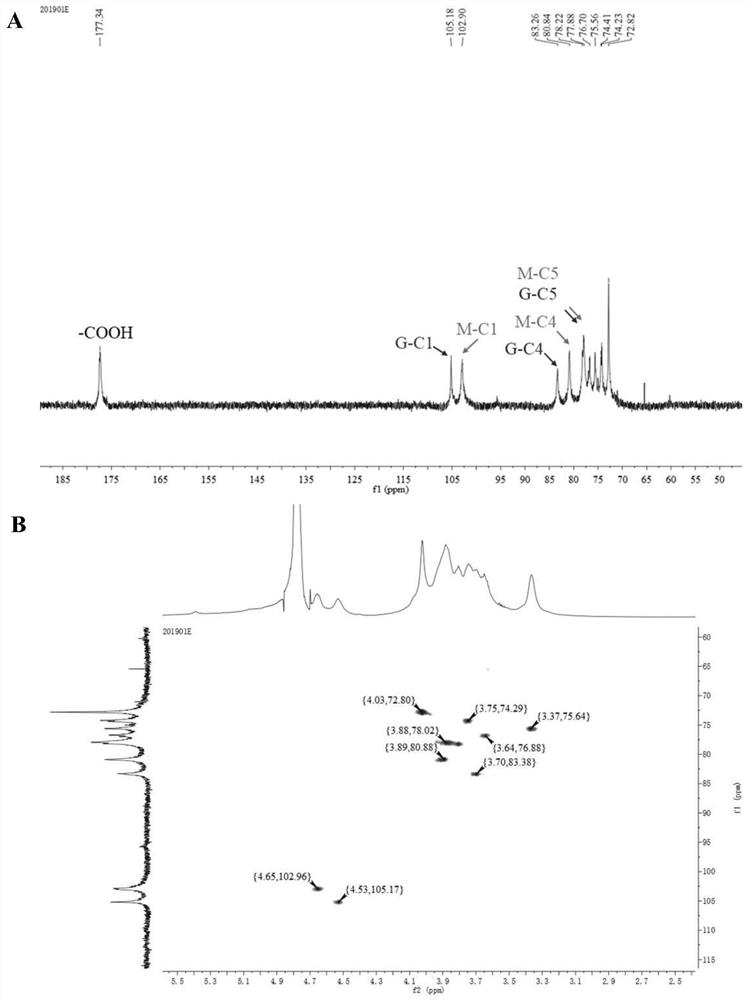
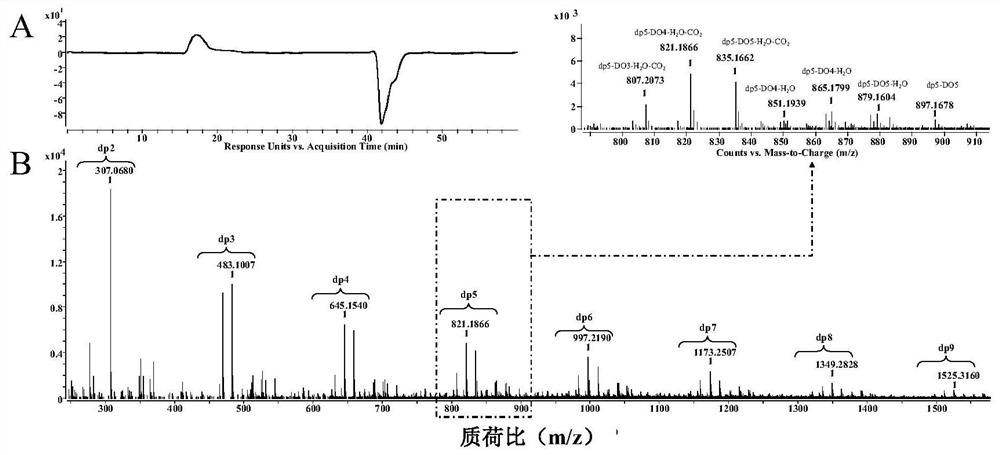
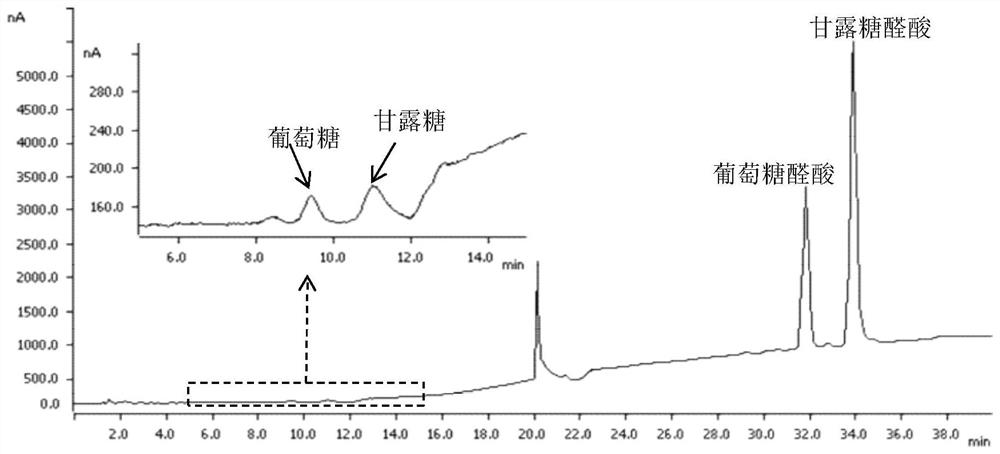
[0050] Preparation process of β-1,4 type glucuronic acid / mannuronic acid linear hybrid oligosaccharides:
[0051] Step 1: Weigh 1g konjac glucomannan, add 192mg 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl radical and 1360mg sodium chlorite successively, then add 200mL. .2 M sodium acetate / acetate buffer solution (pH 4.7), stir well.
[0052] Step 2: Add 4 mL of 5% sodium hypochlorite solution to the solution in step 1, carry out the oxidation reaction at 25°C, and add 5 mL of absolute ethanol after 48 hours to terminate the reaction.
[0053] Step 3: Place the sample obtained above in a 500Da dialysis bag and dialyze for 72 hours to remove salt, filter through a rapid filter membrane to remove insoluble matter, remove the clear liquid from the lower layer, concentrate by rotary evaporation, and freeze-dry to obtain a β-1,4-type glucosin Acid / mannuronic acid linear hybrid polysaccharide solid matter.
[0054] Step 4: Dissolve the above-mentioned polysaccharide in 100 mM ...
Embodiment 2
[0093] Preparation method of β-1,4 type glucuronic acid / mannuronic acid linear hybrid oligosaccharides:
[0094] Step 1: Weigh 10g konjac glucomannan, add 3840mg 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl radical and 2720mg sodium chlorite successively, then add 2000mL. .2M sodium acetate / acetic acid buffer solution (pH 4.7), stir well.
[0095] Step 2: Add 50 mL of 5% sodium hypochlorite solution to the solution in step 1 to carry out the oxidation reaction at 25° C. After 72 hours, add 30 mL of absolute ethanol to terminate the reaction.
[0096] Step 3: Place the sample obtained above in a 500Da dialysis bag and dialyze for 72 hours to remove salt, filter through a rapid filter membrane to remove insoluble matter, take the lower clear liquid, concentrate by rotary evaporation, and freeze-dry to obtain β-1,4-type glucuronic acid / Mannocuronic acid straight-chain hybrid polysaccharide solid matter.
[0097] Step 4: Dissolving the above-mentioned polysaccharide in 1M t...
Embodiment 3
[0101] A preparation method of β-1,4 type glucuronic acid / mannuronic acid linear hybrid oligosaccharides:
[0102] Step 1: Weigh 2g konjac glucomannan, add 192mg 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl radical and 1360mg sodium chlorite successively, then add 200mL. .2 M sodium acetate / acetate buffer solution (pH 4.7), stir well.
[0103] Step 2: Add 12 mL of 5% sodium hypochlorite solution to the solution in step 1 to carry out the oxidation reaction at 25° C. After 96 hours, add 10 mL of absolute ethanol to terminate the reaction.
[0104] Step 3: Place the sample obtained above in a 500Da dialysis bag and dialyze for 72 hours to remove salt, filter through a rapid filter membrane to remove insoluble matter, take the lower clear liquid, concentrate by rotary evaporation, and freeze-dry to obtain β-1,4-type glucuronic acid / Mannocuronic acid straight-chain hybrid polysaccharide solid matter.
[0105] Step 4: Dissolve the above-mentioned polysaccharides in 100mM tri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com