Synthesis method of 2-aminoindan or derivative thereof
A synthetic method, the technology of aminoindane, applied in the field of organic compound synthesis, can solve the problems of harsh conditions, cumbersome operations, and long steps, and achieve the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
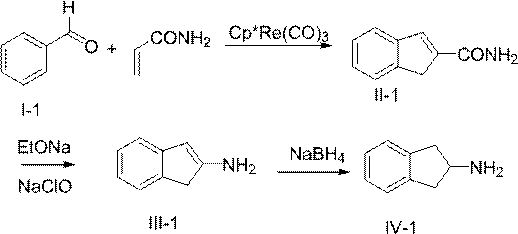
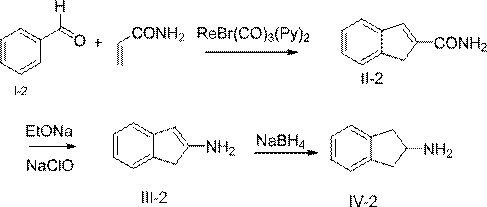
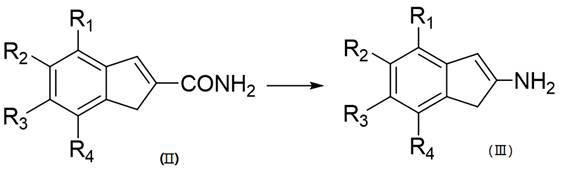
[0033] The invention relates to a method for synthesizing 2-aminoindane or derivatives thereof, comprising the following steps S10-S30.
[0034] Step S10: The compound of formula (I) is cyclized with acrylamide to obtain the compound of formula (II).
[0035]
[0036] Step S20: subjecting the compound of formula (II) to Hofmann degradation reaction to obtain the compound of formula (III).
[0037]
[0038] Step S30: reducing the compound of formula (III) to obtain 2-aminoindan or its derivatives of formula (IV);
[0039]
[0040] Among them, R 1 , R 2 , R 3 and R 4 Each independently selected from: H, C1-C30 alkanyl, C3-C30 cycloalkyl, C1-C30 alkoxy, C6-C30 aromatic hydrocarbon, C6-C30 aromatic alkyl alkoxy or C3 ~C30 heterocyclic aromatic hydrocarbon group.
[0041] Wherein, a C3-C30 cycloalkyl group refers to a cycloalkyl group having 3 to 30 ring atoms.
[0042] The aromatic hydrocarbon groups of C6-C30 include groups formed by dehydrogenation of benzene, na...
Embodiment 1
[0077]
[0078] (1) Catalyst Cp*Re(CO) 3 Synthesis
[0079] According to the literature method, 6.88g pentamethylcyclopentadiene and 10g Re 2 (CO) 10 Put it into a 100mL round bottom flask, heat the oil bath to reflux to 150°C, keep it warm for 0.5h, then slowly raise the temperature to 210°C within 2~5h, keep it warm until no gas is produced, and cool down to room temperature while stirring. Solid precipitated out. Frozen n-hexane beating, recrystallization to obtain the product.
[0080] 1 H NMR (δ, CDCl 3 ) δ 2.21 (s, 15H);
[0081] 13 C NMR (ppm, acetone-d 6 ) 199.1 (CO), 99.6 (C 5 Me 5 ), 10.7(C 5 Me 5 )
[0082] (2) Synthesis of 2-amidoindene (compound II-1)
[0083] Dissolve 10.6g benzaldehyde, 7.1g acrylamide in 500mL toluene, add catalyst Cp*Re(CO) 3 0.12g and 1.84g of p-methoxyaniline, the reaction temperature was raised to 130°C, and the reaction was refluxed for 12h. After the reaction was completed, the temperature was cooled to room temperatur...
Embodiment 2
[0093]
[0094] (1) Catalyst ReBr(CO) 3 (py) 2 Synthesis
[0095] 0.1g ReBr(CO) 5 in N 2After heating to 120°C in the atmosphere, add excess py (5 eq) and react for 1 hour until no bubbles are generated. After cooling down to room temperature, wash the excess py three times with ether, dissolve the solid in chloroform, freeze and crystallize, filter, wash and dry to obtain the product 0.95 g. Yield 77%.
[0096] Elemental analysis: theoretical value: C 33.6 H 2.2 N 6.0 actual value: C 33.1 H 1.9 N 6.4
[0097] 1 H NMR (δ, CDCl 3 ) δ 7.36 (m, 4H), δ 8.59 (m, 4H), δ 8.98 (m, 2H);
[0098] 13 C NMR (ppm, acetone-d 6 ) 199.1 (CO), 149.9 (Py), 137.8 (Py), 121.8 (Py).
[0099] (2) Synthesis of 2-amidoindene (compound II-2)
[0100] Dissolve 10.6g benzaldehyde and 7.1g acrylamide in 500mL toluene, add catalyst ReBr(CO) 3 (py) 2 0.2g and 1.84g of p-methoxyaniline, the reaction temperature was raised to 130°C, and the reaction was refluxed for 12h. After the reaction ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com