Application of stem cell conditioned medium in preparation of medicine for treating inflammatory skin
A conditioned medium and technology for inflammatory skin diseases, applied in the field of biomedicine, can solve the problems of poor quality inflammatory skin effects, etc., and achieve significant technological progress, inhibit lesion, and inhibit skin inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Preparation of human umbilical cord mesenchymal stem cell conditioned medium for skin medicine (UMSCM)
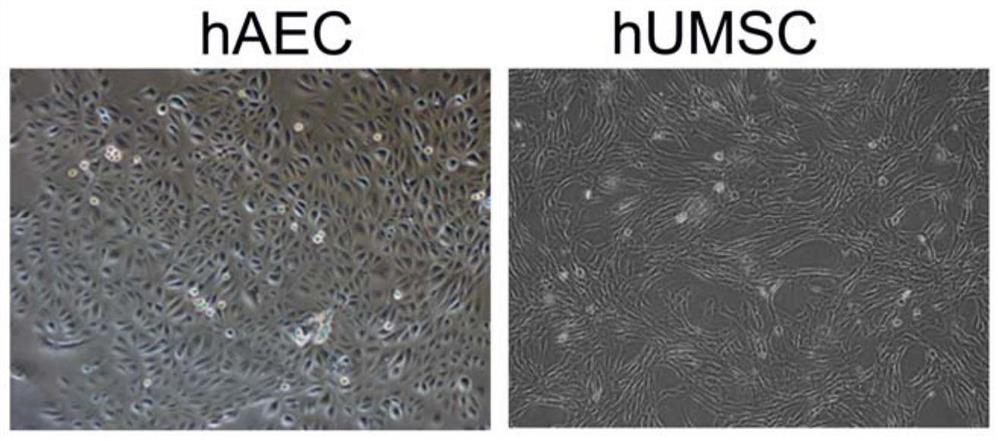
[0035] 1) Isolation and culture of human umbilical cord mesenchymal stem cells: After cleaning the human umbilical cord with sterile saline, remove the umbilical artery and umbilical vein blood vessels with tooth forceps, and tear off the remaining tissue after the epidermis, cut into Tissue blocks with a size of 3mm, and then, adopt the method of tissue climbing, let the tissue stick in the culture dish, add the medium alpha-MEM containing the platelet lysate with a concentration of 5% by volume. The medium was changed slowly after 7 days, and the cells crawled out of the tissue block after about 2 weeks. Subculture was carried out when the cells reached 90% density, and the morphology of umbilical cord mesenchymal stem cells was shown in figure 1 .
[0036] 2) Collect stem cell conditioned medium: continue to culture umbilical cord mesenchymal stem cel...
Embodiment 2
[0038] Example 2: Preparation of human amnion epithelial cell conditioned medium for skin medication (AECM)
[0039] 1) Isolation and culture of amniotic epithelial stem cells: Isolate the amniotic membrane from human placenta tissue, wash it with sterile saline, separate the epithelial layer, the layer close to the fetus, and then digest it with 0.25% trypsin for 30 Minutes, and then digested with 0.1g / L collagenase for 1 hour, finally, filtered and centrifuged to obtain primary amniotic epithelial stem cells, in DMEM / F12 medium containing 5% platelet lysate by volume, when cells were found Colony growth, when the adherent cells reached 80%-90% confluence, they were digested with 0.25% trypsin in mass percentage concentration, and then subcultured; the morphology characteristics of amniotic membrane epithelial stem cells were as follows figure 1 shown.
[0040] 2) Collect stem cell conditioned medium: continue to culture umbilical cord mesenchymal stem cells or amniotic memb...
Embodiment 3
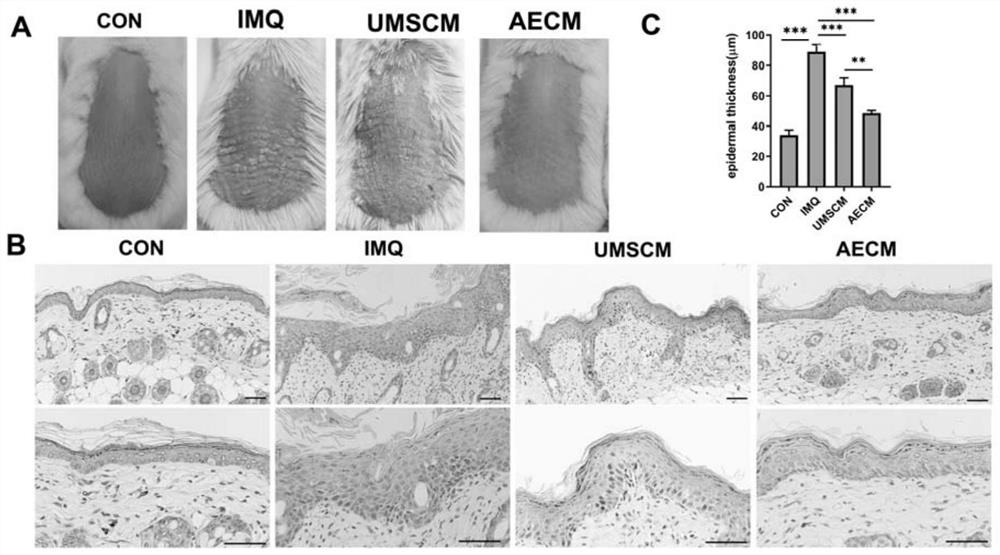
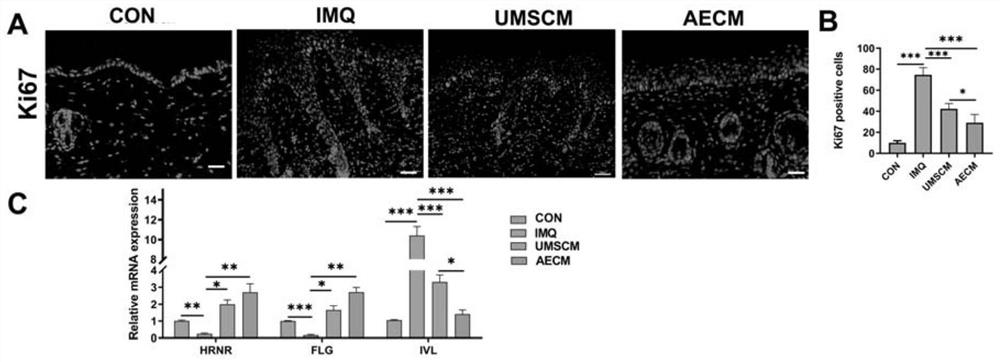
[0042] Example 3: Quality identification of human umbilical cord mesenchymal stem cells and human amniotic epithelial cell conditioned medium
[0043] 1) The stem cell conditioned medium is subjected to sterility testing, detection of mycoplasma, endotoxin, etc.
[0044] 2) The stem cell conditioned medium was subjected to independent and allergy testing experiments.
[0045] 3) BCA quantification is performed on the stem cell conditioned medium, and the total protein amount and protein quantification of important factors in it are determined. Specifically, the content detection of active ingredients IL-1ra, IL-10, KGF, bFGF, for IL-1ra content is 400-1000pg / ml, IL-10 is 50-200pg / ml, KGF, EGF, bFGF and content are 50-200pg / ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com