Method for preparing p-phenylene diisocyanate by non-phosgene method
A technology of p-phenylene diisocyanate and p-phenylene diamine, which is applied in the field of non-phosgenation preparation of p-phenylene diisocyanate, and achieves the effects of short reaction time, simple product separation and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
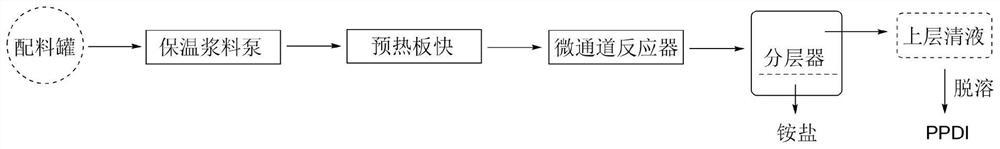
[0071] A method for preparing p-phenylene diisocyanate by non-phosgene method, using N,N"-(1,4-phenylene)bis(N',N'-diethyl)urea (hereinafter referred to as "p-phenylene diisocyanate) Diamine diethyl urea") is a raw material, methanesulfonic acid is a protonic acid, and adopts a batch tank type reactor to generate p-phenylene diisocyanate by one-step pyrolysis. Specifically,
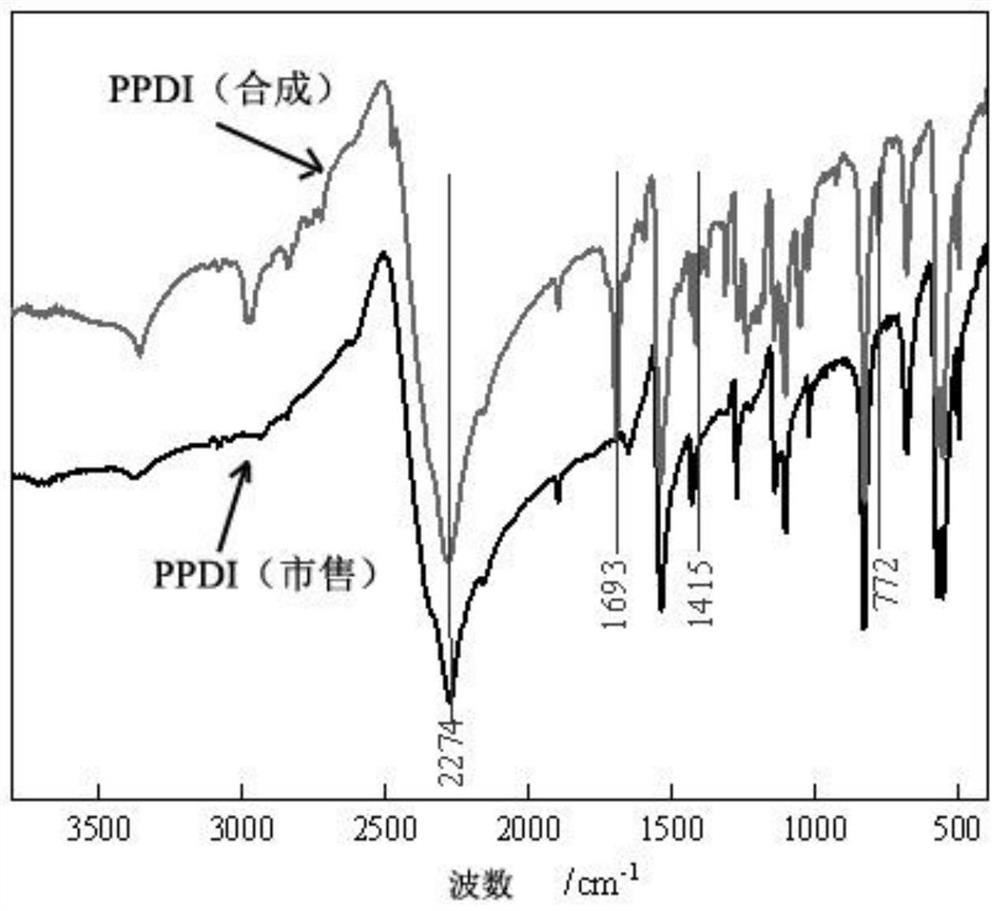
[0072] Weigh a quantitative amount of p-phenylenediamine diethylurea into a 100ml two-necked flask, add an appropriate amount of solvent (cyclohexane, xylene, trimethylbenzene or n-octane), heat and stir to a given temperature (80-170°C within the range). Then weigh quantitative methylsulfonic acid and add it to the reaction flask, react at a constant temperature (within 1 to 4 hours), observe the reaction phenomenon, and use infrared spectroscopy to track the reaction. -1 The absorption peak of NCO appeared at , indicating the formation of the product. After the reaction liquid is lowered to room tempe...
Embodiment 2
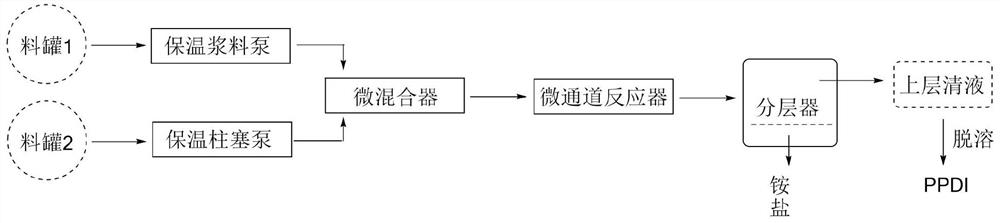
[0082] A method for preparing p-phenylene diisocyanate by non-phosgene method, using N,N"-(1,4-phenylene)bis(N',N'-diisopropyl)urea (hereinafter referred to as "p-phenylene diisocyanate") Phenylene diisopropyl urea ") is raw material, and n-octane is solvent, and methanesulfonic acid is protonic acid, adopts batch still reactor one-step pyrolysis to generate p-phenylene diisocyanate. Experimental procedure is identical with embodiment 1.
[0083] The consumption, reaction conditions and productive rate of reaction raw material, protonic acid and solvent in table 2 embodiment 2
[0084]
[0085] Experimental phenomena and results:
[0086] (1) P-phenylenediisopropylurea is insoluble in n-octane at room temperature, and it will gradually dissolve when heated to 125°C. During the reaction, a colorless viscous liquid is produced at the bottom of the reactor, and the colorless viscous liquid becomes white solid;
[0087] (2) Infrared spectrum tracking reaction, the absorption ...
Embodiment 3
[0091] A method for preparing p-phenylene diisocyanate by non-phosgene method, using N,N"-(1,4-phenylene) bis(N',N'-diisopropyl)urea ("p-phenylene diisopropyl) urea") as raw material, n-octane as solvent, and hydrogen chloride as protonic acid. Weigh quantitative p-phenylenediisopropylurea (2.263g, 6.25mmol), put it into a 100ml two-necked flask at room temperature, add n-octane (50ml), obtain the incompletely dissolved slurry.In the 50ml there-necked flask, ethanol (5.75g, 0.125mol) is loaded into, after feeding nitrogen flow, start to add tetrachlorosilane (5.31g, 31.25mmol) dropwise, will produce The hydrogen chloride gas was passed to the bottom of the above-mentioned 100ml reaction bottle solution, stirred at the same time, and then gradually heated up to 115°C. During the reaction, stratification occurred. After 4 hours of reaction, it was lowered to room temperature, and stood for 2 hours, and a white solid was produced at the bottom. Filtration to obtain the filtrate, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com