Application of fat active protein in preparation of medicine for treating premature ovarian insufficiency
An active protein and ovarian function technology, which is applied in the application field of lipoactive protein in the preparation of drugs for the treatment of premature ovarian insufficiency, can solve problems such as the inability to fundamentally improve female fertility, avoid the risk of immune rejection and Ethical Controversy, Increased Ovulation Count, Effect of Improving Ovarian Function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Extraction of adipose decellularization active protein (CEFFE):
[0040] Collect adipose tissue from women undergoing liposuction, first rinse with normal saline to remove blood components in the adipose tissue, then centrifuge at 1200g for 3 minutes, remove the upper oil layer and lower liquid layer after centrifugation, and collect the middle fat layer for further mechanical emulsification 30min. The emulsified adipose tissue was frozen in a -80°C refrigerator, and then thawed at 37°C to further destroy the adipose tissue. After a freeze-thaw process, the adipose tissue was centrifuged again at 1200 g for 5 min. After the second centrifugation, the adipose tissue is divided into four layers. The first oil layer, the second unbroken fat layer and the fourth debris layer are discarded, and the third water layer is collected, which is the "fat decellularized active protein layer". , pipette carefully to prevent contamination from sedimentation. Finally, the collected fa...
Embodiment 2
[0042] Animal model construction and treatment plan for chemotherapy-induced premature ovarian insufficiency:
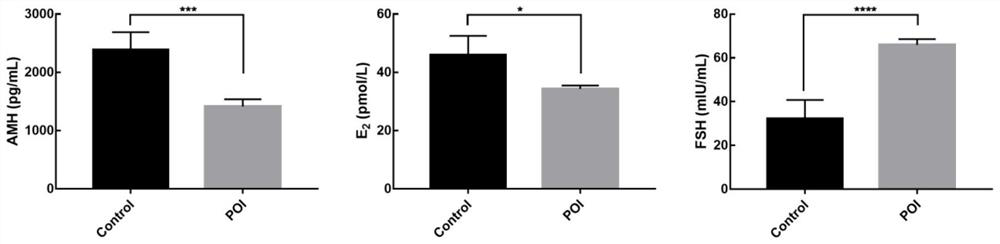
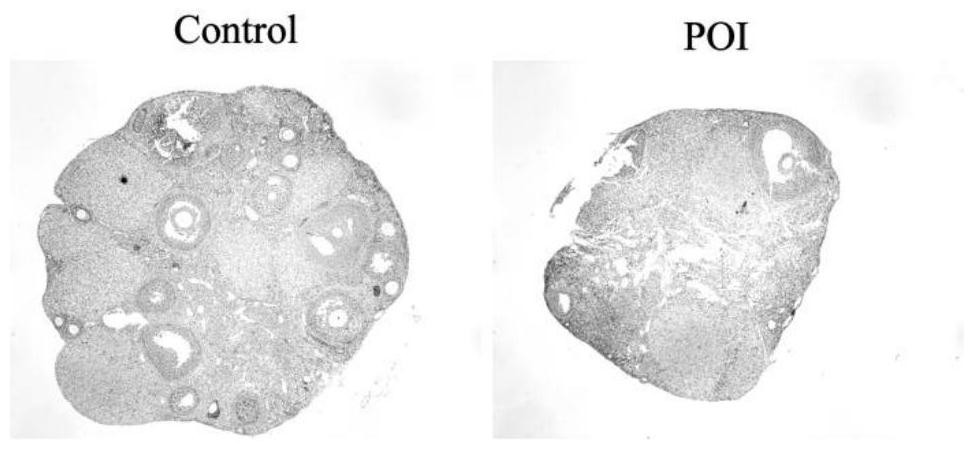
[0043] A 6-8 week-old C57BL / 6N female mouse experimental animal model was selected, and the feeding conditions were room temperature (25±2° C.), humidity 45-55%, and light time 12 hours. The mice were randomly divided into blank control group (Control), disease group (POI), and treatment group (POI+CEFFE), 40 in each group. Firstly, the disease model was constructed for POI group and POI+CEFFE group, that is, each mouse was given intraperitoneal injection of cyclophosphamide (CTX) 120 mg / kg + busulfan (BUS) 12 mg / kg, and the above drugs were dissolved in DMSO. 14 days after modeling, the body weight of the mice was observed, and the serum sex hormones anti-Müllerian hormone (AMH), estradiol (E 2 ), gonadotropin (FSH) content, and the number of follicles observed in the pathological morphology of the ovary, and the above indicators were used to confirm whether the PO...
Embodiment 3
[0049] Mouse body weight and estrous cycle detection:
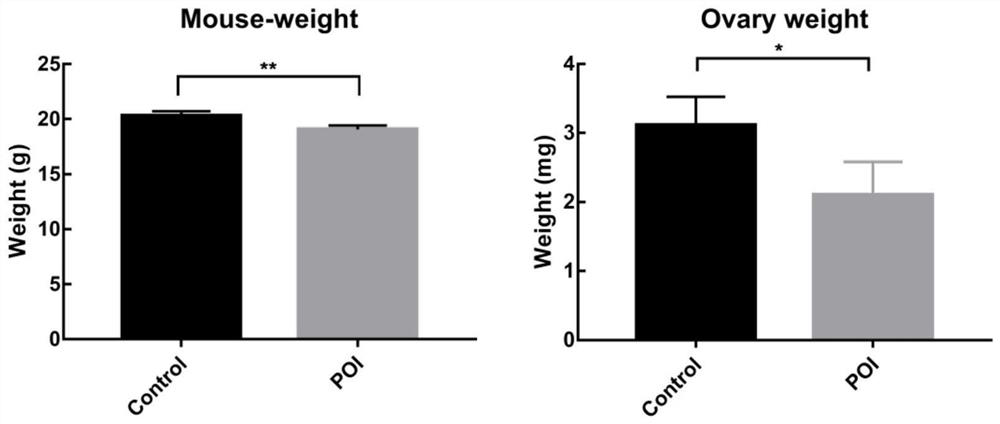
[0050] After administration of chemotherapeutic drugs to the mice to establish the model in Example 2, the body weight of the mice was continuously detected every day until the end of the treatment. like Figure 4 It can be seen that after the mice were given chemotherapy drugs to create models, compared with the Control group, the body weight of the mice in the POI group decreased significantly after the model was established, and after the treatment with CEFFE, the body weight of the mice increased significantly compared with the POI group. The weight of the ovaries in the POI group was significantly lower than that in the Control group, and after treatment with CEFFE, the weight of the ovaries in the mice increased significantly (P<0.05).
[0051] The vaginal secretions were collected at 8Am every day, and then the secretion smears were taken to observe the changes in the estrous cycle. The specific sampling method wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com