Preparation method and application of NiFe-LDH catalyst for reducing nitrate into ammonia
A catalyst and nitrate technology, applied in the direction of catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of not improving the selectivity of nitrate into ammonia, low catalytic performance, etc., and achieve product form Good appearance, high specific surface area, mild condition effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0032] 1. Preparation of NiFe-LDH catalyst
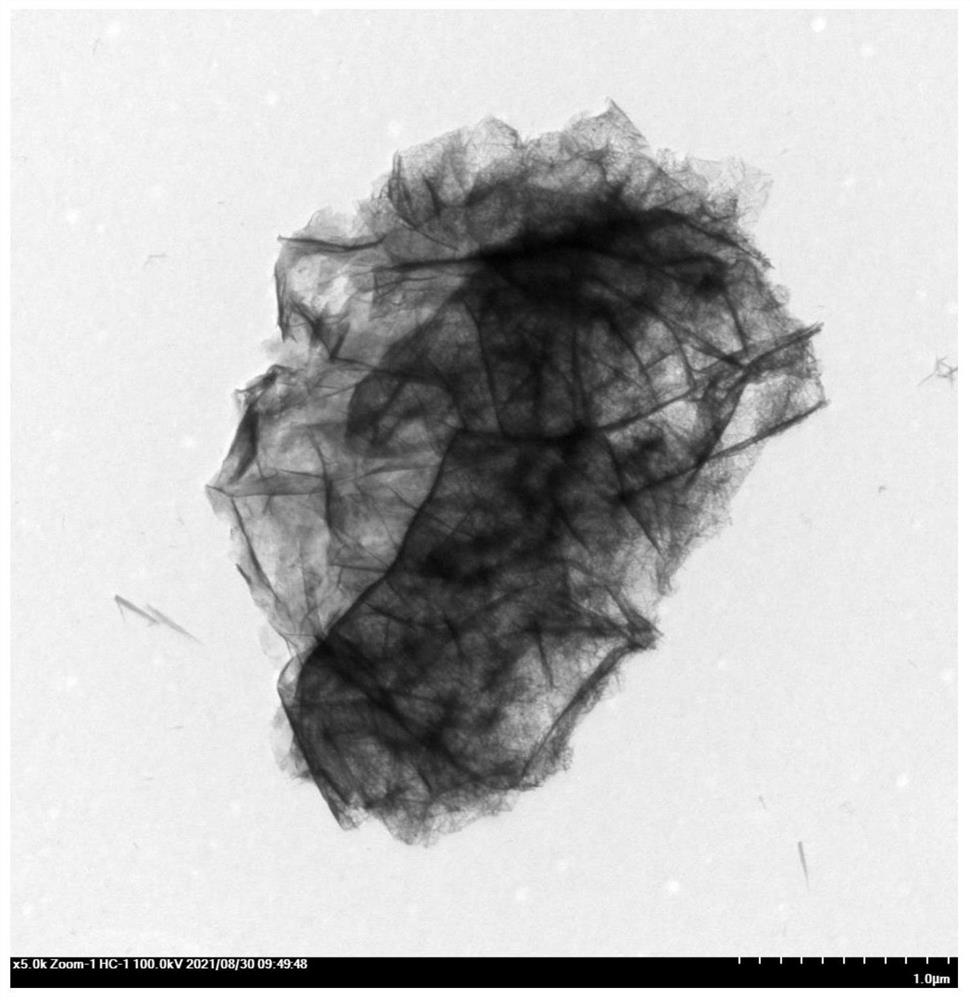
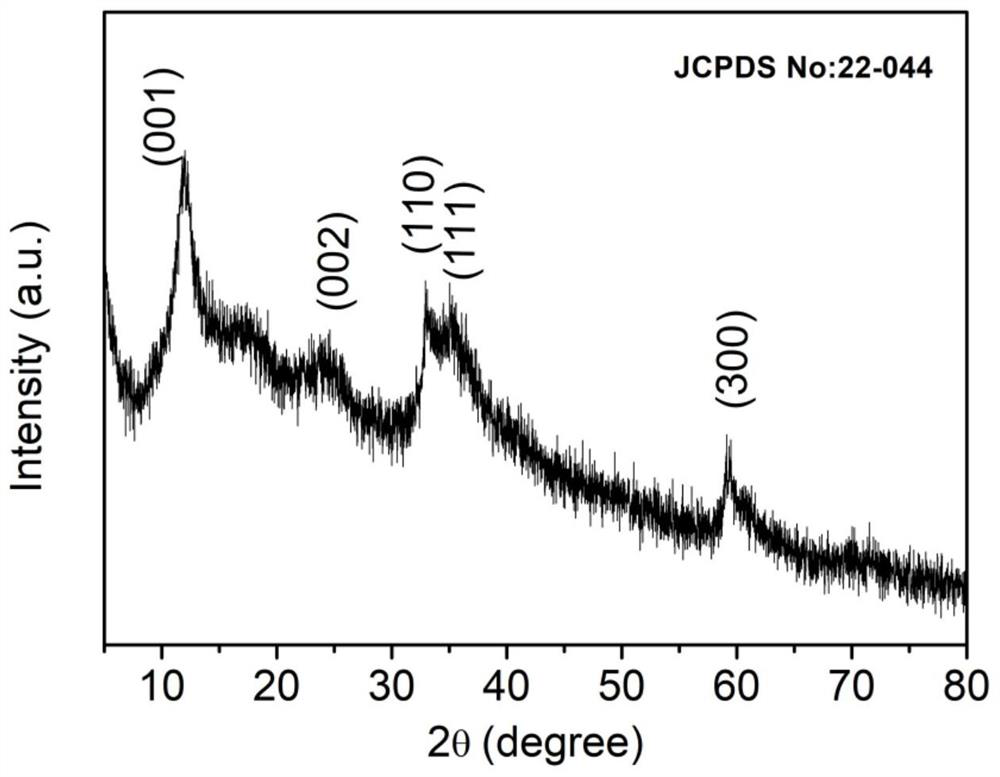
[0033]Dissolve nickel nitrate hexahydrate and urea in the mixed solution of water and triethylene glycol, stir for 8 to 10 minutes, then add ferric trichloride hydrate, and continue stirring for 30 to 40 minutes, wherein nickel nitrate hexahydrate and trichloride hydrate The molar ratio of iron to nickel and iron is 1:0.1~1; then hydrothermally react at 110~130°C for 20~26h; after the reaction, wash and dry sequentially to obtain NiFe-LDH catalyst.
[0034] 2. Reactivity test of NiFe-LDH catalyst
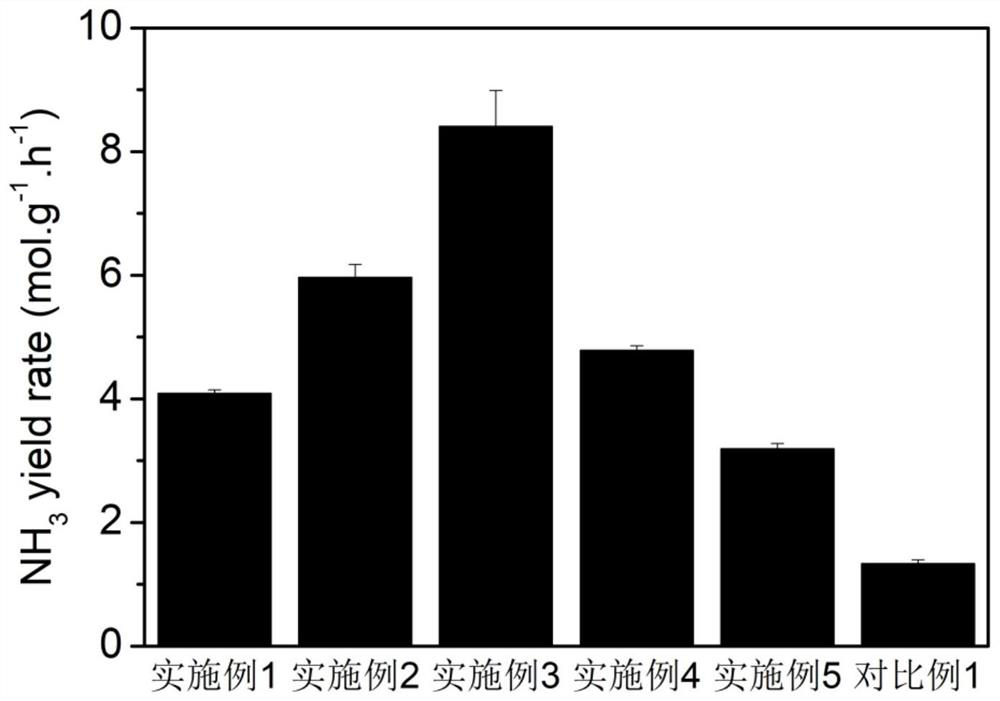
[0035] Add 4mg of NiFe-LDH catalyst to a mixed solution of 750uL deionized water, 200uL isopropanol and 50uL naphthol to make a catalyst solution, and drop 30uL onto 1cm*1cm carbon paper as the cathode. Using a three-electrode system, the carbon paper was clamped by the electrode holder as the working electrode, the silver / silver chloride electrode was used as the reference electrode, and the platinum mesh was used as the counter electro...
Embodiment 1
[0041] 1. Preparation of NiFe-LDH catalyst
[0042] Dissolve nickel nitrate hexahydrate and urea in a mixed solution of 50 mL deionized water and triethylene glycol, stir for 10 minutes, then add ferric chloride hydrate, and continue stirring for 30 minutes, wherein nickel nitrate hexahydrate and ferric chloride hydrate The molar ratio of nickel and iron is 1:0.5; followed by hydrothermal reaction at 120°C for 24 hours; after the reaction, washed with deionized water and absolute ethanol, dried in a vacuum oven to obtain NiFe-LDH catalyst .
[0043] 2. Reactivity test of NiFe-LDH catalyst
[0044] Add 4mg of NiFe-LDH catalyst to a mixed solution of 750uL deionized water, 200uL isopropanol and 50uL naphthol to make a catalyst solution, and drop 30uL onto 1cm*1cm carbon paper as the cathode. Using a three-electrode system, the carbon paper was clamped by the electrode holder as the working electrode, the silver / silver chloride electrode was used as the reference electrode, and...
Embodiment 2
[0046] 1. Preparation of NiFe-LDH catalyst
[0047] Dissolve nickel nitrate hexahydrate and urea in a mixed solution of 50 mL deionized water and triethylene glycol, stir for 10 minutes, then add ferric chloride hydrate, and continue stirring for 30 minutes, wherein nickel nitrate hexahydrate and ferric chloride hydrate The molar ratio of nickel to iron is 1:0.4; followed by hydrothermal reaction at 120°C for 24 hours; after the reaction, washed with deionized water and absolute ethanol, dried in a vacuum oven to obtain NiFe-LDH catalyst .
[0048] 2. Reactivity test of NiFe-LDH catalyst
[0049] Add 4mg of NiFe-LDH catalyst to a mixed solution of 750uL deionized water, 200uL isopropanol and 50uL naphthol to make a catalyst solution, and drop 30uL onto 1cm*1cm carbon paper as the cathode. Using a three-electrode system, the carbon paper was clamped by the electrode holder as the working electrode, the silver / silver chloride electrode was used as the reference electrode, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com