Method for preparing Daptomycin impurity RS-7a
A technology of daptomycin and rs-7a, applied in the field of preparation of daptomycin impurity RS-7a, can solve the problems of undisclosed impurity molecular structure or molecular weight, difficult operation, large consumption and flow, etc., and achieves economical preparation cost, control the production process, and improve the effect of product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
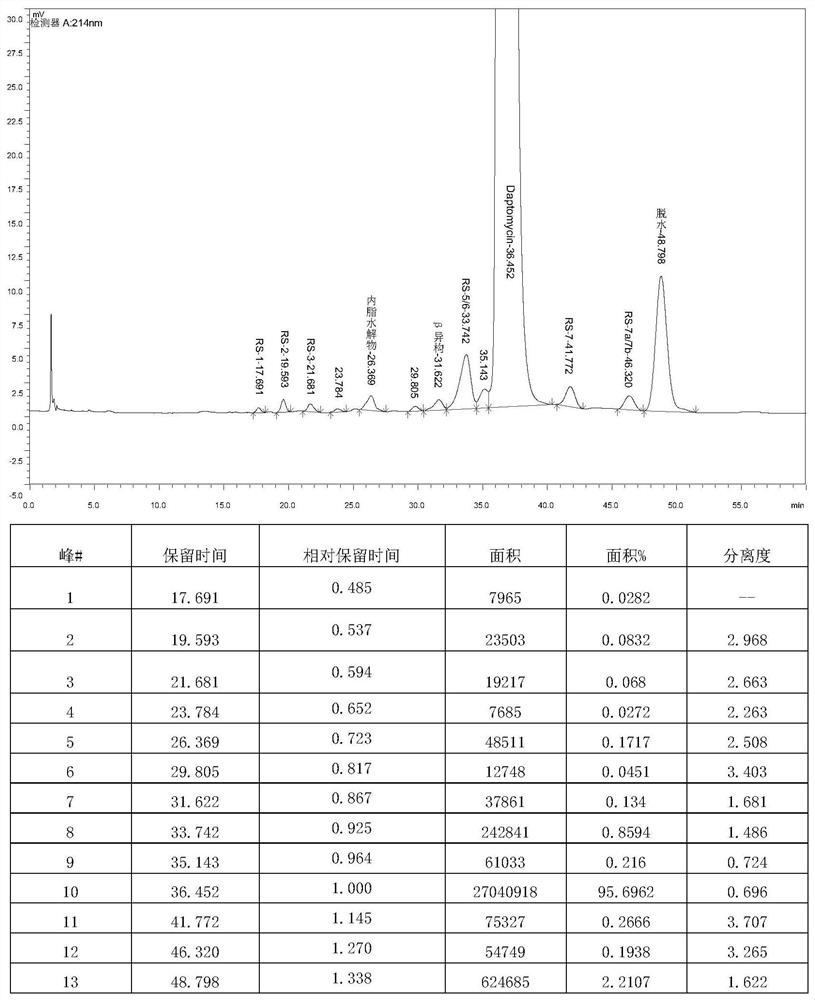
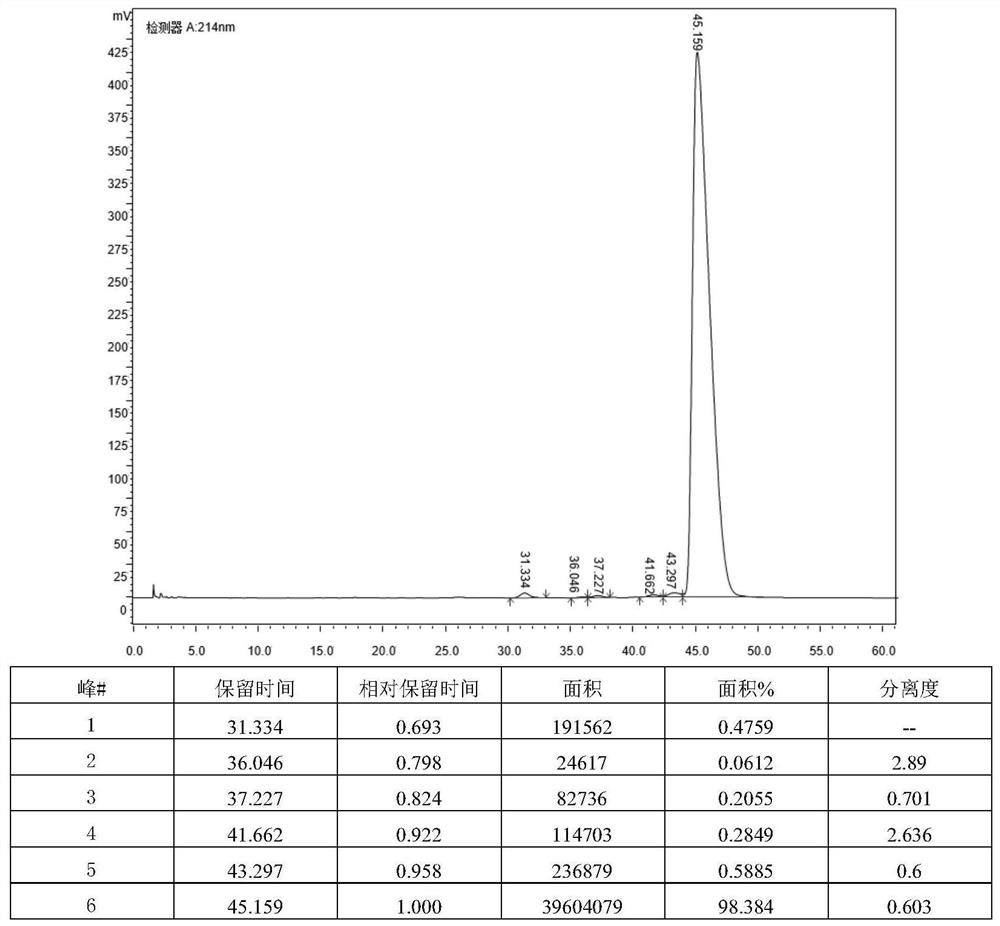
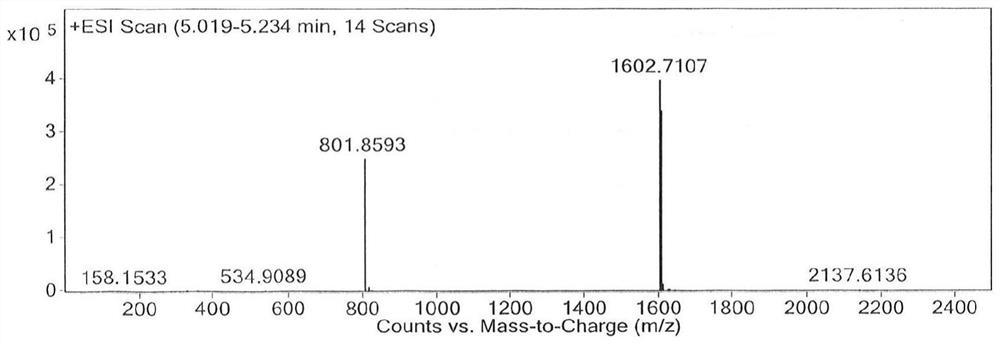
[0055] Get the finished daptomycin product (5g) with a chromatographic purity of 95%, and dissolve it into a 40mg / mL solution with a volume ratio of 35% acetonitrile-water solution, then add 1mol / L hydrochloric acid solution to the acetonitrile-water solution containing daptomycin , the pH of the reaction solution was adjusted to 4.1, placed in a 50°C constant temperature water bath, heated for 72 hours to obtain a solution after the acid reaction, and the purity of the impurity RS-7a in the solution after the acid reaction was detected by HPLC to be 9.8%; then the acid reaction The final solution is separated and purified through preparative chromatography (the chromatographic column is LP-C8, 250 × 21.2mm), and the mobile phase is a mixed solution of acetonitrile and 0.03mol / L ammonium dihydrogen phosphate solution (the volume ratio of acetonitrile and ammonium dihydrogen phosphate solution 35:65, the pH of the ammonium dihydrogen phosphate solution is 4.0), the loading flow ...
Embodiment 2
[0057] Take daptomycin powder (2g), dissolve it into a 50mg / mL solution with a volume ratio of 30% acetonitrile-water solution, then add 1mol / L hydrochloric acid solution to the acetonitrile-water solution containing daptomycin to make the reaction solution Adjust the pH to 3.5, place it in a constant temperature water bath at 40°C, heat and react for 76 hours to obtain a solution after the acid reaction, and the purity of the impurity RS-7a in the solution after the acid reaction was detected by HPLC to be 9.6%; then the solution after the acid reaction was passed through Preparative chromatography (the chromatographic column is LP-C8, 250 × 21.2mm) separation and purification, the mobile phase is a mixed solution of acetonitrile and 0.04mol / L ammonium dihydrogen phosphate solution (the volume ratio of acetonitrile and ammonium dihydrogen phosphate solution is 30:70 , the pH of the ammonium dihydrogen phosphate solution is 3.5), the loading flow rate of the mobile phase is 15m...
Embodiment 3
[0059] Get the finished daptomycin product (5g) with a chromatographic purity of 95%, dissolve it into a 30mg / mL solution with a volume ratio of 40% acetonitrile-water solution, then add 0.1mol / L sulfuric acid to the acetonitrile-water solution containing daptomycin solution, the pH of the reaction solution was adjusted to 4.5, placed in a 65°C constant temperature water bath, heated for 68 hours to obtain a solution after the acid reaction, and the purity of the impurity RS-7a in the solution after the acid reaction was detected by HPLC to be 9.5%; then the acid The solution after the reaction is separated and purified through preparative chromatography (the chromatographic column is LP-C8, 250 × 21.2mm), and the mobile phase is a mixed solution of acetonitrile and 0.02mol / L ammonium dihydrogen phosphate solution (the volume of acetonitrile and ammonium dihydrogen phosphate solution The ratio is 30:70, the pH of the ammonium dihydrogen phosphate solution is 4.5), the loading f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com