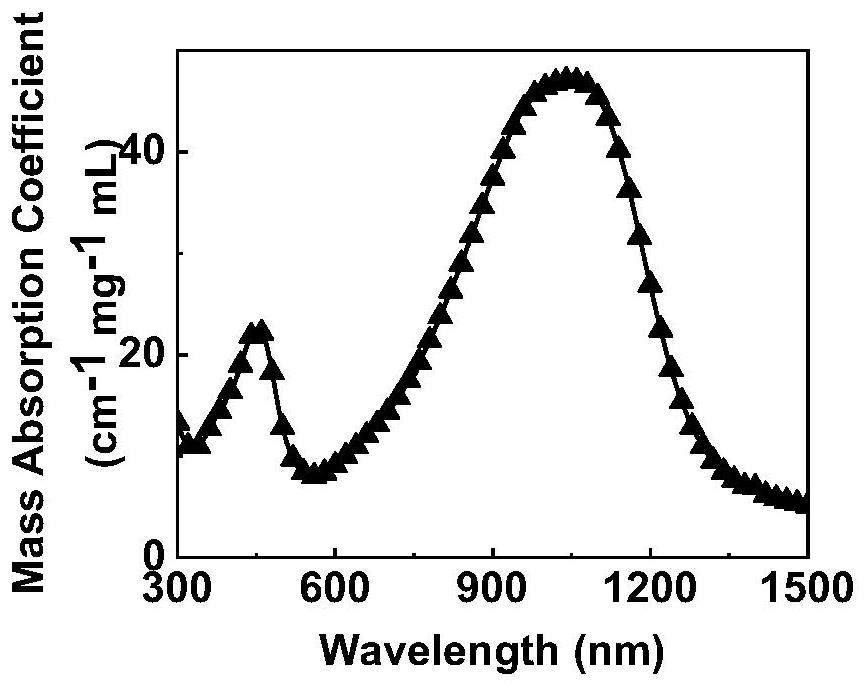
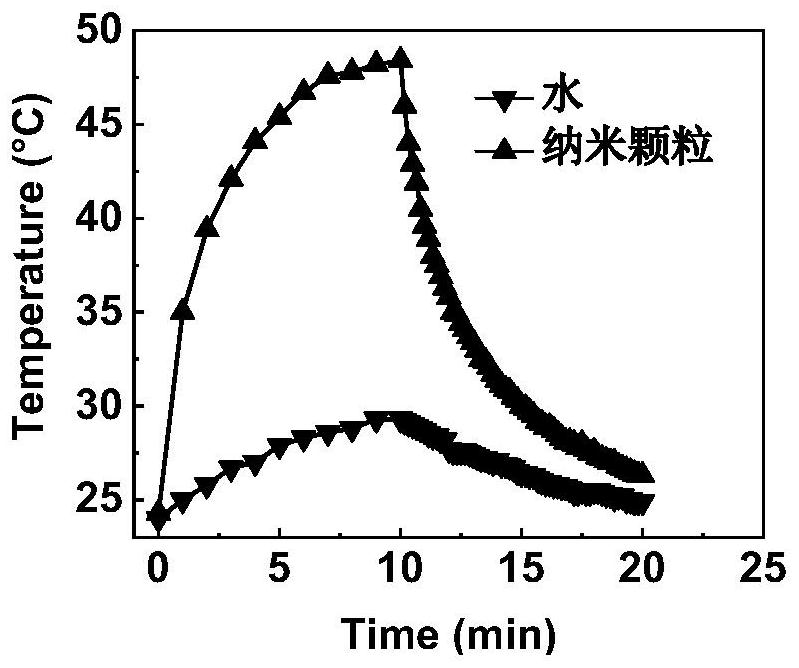
Semiconductor polymer for treating hypoxic tumors, preparation method and application
A semiconductor and polymer technology, applied in the field of nanomedicine, can solve the problems that there are no organic/polymer photosensitizers in NIR-III type PDT/PTT, and achieve the effects of improving photothermal conversion efficiency, promoting production, and strong absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The synthesis steps of semiconducting polymers for the treatment of hypoxic tumors are as follows:
[0039] Step 1: According to the procedure described in the literature (Adv. Funct. Mater. 2013, 23, 5317), synthesize the bilateral iodide of thiophene isoindigo.
[0040] Step 2: According to the procedure described in the literature (Angew.Chem.Int.Ed.2014, 53, 10691), a double-sided tin compound of tellurophene was synthesized.
[0041] Step 3: Under the protection of nitrogen, the bilateral iodide ((E)-2,2'-diiodo-4,4'-bis(2-octyldodecyl)-[6,6'-bithieno[3, 2-b]pyrrolylidene]-5,5'(4H,4'H)-dione) (0.05 mmol) and bistannide of tellurphene (0.05 mmol) were added to a Schlenk reaction flask. After the reaction flask was degassed and refilled with nitrogen for 3 times, dry toluene (2.0 mL) was added, and nitrogen was bubbled for 15 minutes. Then, add Pd under the protection of nitrogen 2 (dba) 3 (0.0025mmol) and tris(o-methylphenyl)phosphorus (0.010mmol). The solution...
Embodiment 2
[0045] The synthesis of semiconducting polymers for the treatment of hypoxic tumors is as follows:
[0046] Step 1: According to the procedure described in the literature (Polym.Chem.2016, 7, 1181), synthesize the double-sided tin compound of thiophene isoindigo.
[0047] Step 2: According to the procedure described in the literature (Organometallics 2016, 35, 2140), the bilateral bromide of tellurocyclohexane was synthesized.
[0048] Step 3: Under the protection of nitrogen, the double-sided tin compound of thiophene isoindigo ((E)-4,4'-bis(2-hexyldecyl)-2,2'-bis(trimethylstannyl)-[6,6'-bithieno [3,2-b]pyrrolylidene]-5,5'(4H,4'H)-dion) (0.05 mmol) and tellurocyclohexane bis bromide (0.05 mmol) were added to a Schlenk reaction vial. After the reaction flask was degassed and refilled with nitrogen for 3 times, dry toluene (2.0 mL) was added, and nitrogen was bubbled for 15 minutes. Then, add Pd under the protection of nitrogen 2 (dba) 3 (0.0025mmol) and tris(o-methylphenyl...
Embodiment 3
[0052] Step 1: According to the procedure described in the literature (Polym.Chem.2016, 7, 1181), synthesize the double-sided tin compound of thiophene isoindigo.
[0053] Step 2: According to the procedure described in the literature (Angew.Chem.Int.Ed.2010, 49, 10140), synthesize the double-sided iodide of bitellurphene.
[0054] Step 3: Under the protection of nitrogen, the double-sided tin compound of thiophene isoindigo ((E)-4,4'-bis(2-hexyldecyl)-2,2'-bis(trimethylstannyl)-[6,6'-bithieno [3,2-b]pyrrolylidene]-5,5'(4H,4'H)-dion) (0.05 mmol) and ditellurene bi-iodide (0.05 mmol) were added to a Schlenk reaction vial. After the reaction flask was degassed and refilled with nitrogen for 3 times, dry toluene (2.0 mL) was added, and nitrogen was bubbled for 15 minutes. Then, Pd2(dba)3 (0.0025 mmol) and tris(o-methylphenyl)phosphorus (0.010 mmol) were added under nitrogen protection. The solution was then bubbled again with nitrogen for 15 minutes. The above mixture was heat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com