Engineering strain for producing recombinant human IL-1ra and preparation method and use thereof
A technology of engineering strains and strains, applied in the field of genetic engineering, can solve the problems of Escherichia coli toxicity, influence on drug safety, process condition control and expression level not as ideal as IPTG, so as to improve drug quality and safety, increase production and The effect of detection cost and reducing the pressure of subsequent purification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
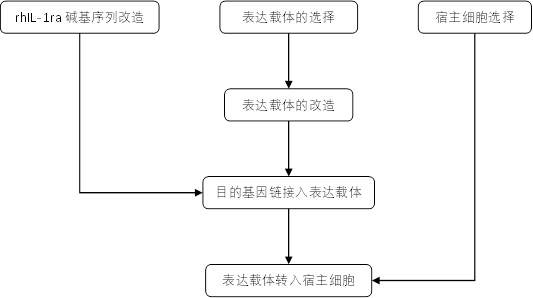
[0078] Example 1 Construction of Engineering Bacteria and Induced Expression of Target Protein
[0079] 1. Base sequence design of IL-1ra
[0080] The original gene sequence was selected as the vector construction.
[0081] The human peripheral blood cDNA library stimulated by PMA was used as a template for PCR amplification. The sequence of the synthetic hIL-1ra primer is shown in SEQ ID NO: 4-5.
[0082] The upstream primer is: 5'-CGACCCTCTGGGAGAAAATCC-3';
[0083] The downstream primer is: 5'-CTTAAGCTTACTCGTCCTCCTGGAAGTGG-3';
[0084] The PCR system is: 10XTE buffer 2μl; DNA polymerase 1-2μl; dNTP 1-2μl; primer 1-2μl; template DNA 1-2μl;
[0085] Taq DNA polymerase, thermocycling conditions: 90°C-95°C 3-5min, 1 cycle; 90°C-95°C 1min-2min, 40°C-60°C 2min-3min, 70°C-75°C 3min-4min, 30 -35 cycles; 70°C-75°C for 10-20min, 1 cycle. The IL-1ra PCR product was purified using the Magic CR purification kit from Promega, USA.
[0086] 2. Carrier transformation
[0087] 1) Cut...
Embodiment 2
[0143] 1. Gene sequence modification
[0144] Select the following mutation sites for genetic modification:
[0145]
[0146] Remove the start codon at the 5' end: atg; add the sequence at the 3' end: gcttagg, and form the restriction site of HandIII with the tail.
[0147] A new sequence is formed as shown in SEQ ID NO:7.
[0148] Due to the large number of mutation sites, the gene sequence was obtained by whole gene synthesis.
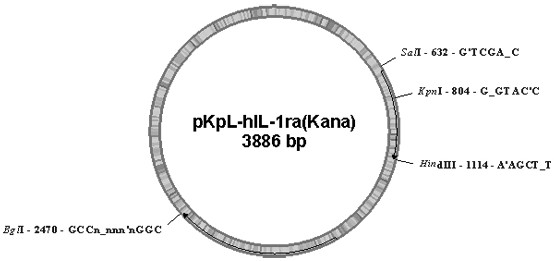
[0149] 2. Transformation of the vector, construction of the pKpL-3K-rhIL-1ra vector, transfer of the vector into host cells and engineered strains to induce expression of the target protein, the same as in Example 1.
[0150] Through the identification of the strain and the expression product, the results are as follows:
[0151] Westen Blot identified that the expression product can bind to IL-1ra antibody, which conforms to the immunological characteristics.
[0152] The N-terminal sequencing results showed that the modified amino acid seque...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com