Protein compound based on Escherichia coli E family DNA enzyme and application of protein compound in artificial protein scaffold
A protein complex and colicin technology, applied in the application field of artificial protein scaffolds, can solve the problems of limited application and achieve the effect of efficient hydrolysis and unified structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
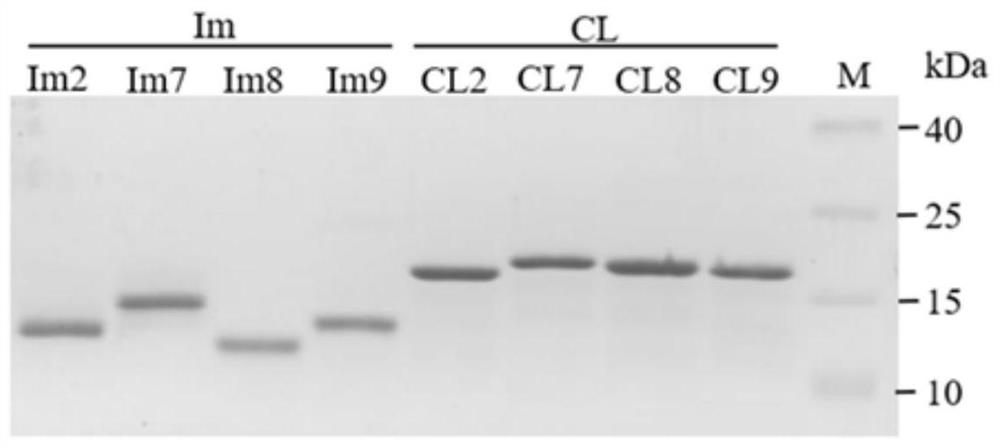
[0043] Example 1 Expression and purification of CL protein and Im protein
[0044] 1. Through amino acid sequence comparison with CL7 protein, based on the high similarity of the four protein sequences, the carboxy-terminal DNase domains of CE family DNases CE2, CE7, CE8 and CE9 were protein engineered to obtain lost DNase activity, but still retain the mutants CL2, CL7, CL8 and CL9 with super high affinity to the corresponding immune protein Im protein, among which the four sets of CL-Im and four wild-type CE protein sequences are:
[0045] (1) CE2 protein: as shown in SEQ ID NO.1; CL2 protein: as shown in SEQ ID NO.2; Im2 protein: as shown in SEQ ID NO.3;
[0046] (2) CE7 protein: as shown in SEQ ID NO.4; CL7 protein: as shown in SEQ ID NO.5; Im7 protein: as shown in SEQ ID NO.6;
[0047] (3) CE8 protein: as shown in SEQ ID NO.7; CL8 protein: as shown in SEQ ID NO.8; Im8 protein: as shown in SEQ ID NO.9;
[0048] (4) CE9 protein: as shown in SEQ ID NO.10; CL9 protein: as s...
Embodiment 2
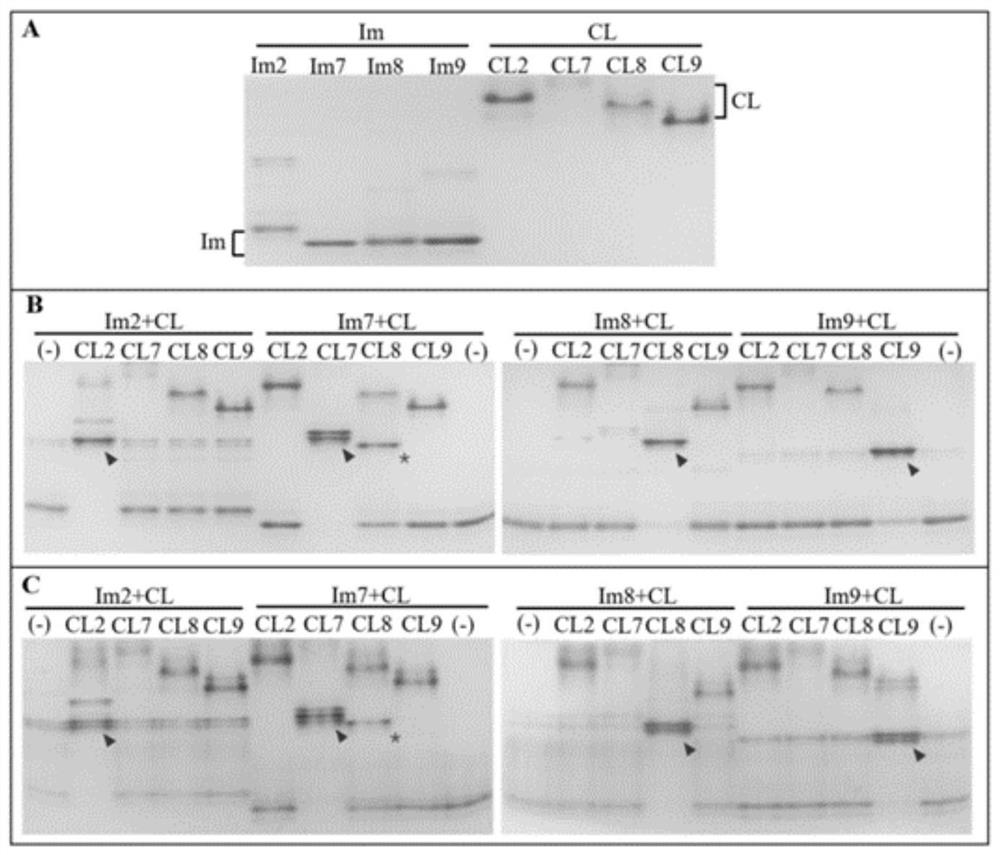
[0051] Example 2 Native-PAGE
[0052] 1. Native-PAGE non-denaturing acrylamide gel preparation
[0053] For the verification of the binding specificity of the CL-Im protein interaction pair, a 30% acrylamide (37.5:1), glycerol electrophoresis system was used, and the specific formula is shown in Table 1.
[0054] Table 1 Native-PAGE non-denaturing acrylamide gel recipe
[0055]
[0056] 2. Native-PAGE
[0057] In order to verify the specificity of the binding between CL protein and Im protein, in this example, 4 kinds of CL proteins and 4 kinds of Im proteins were mixed separately to obtain 16 combinations. After incubation at room temperature for 30 minutes, Native-PAGE was performed. The voltage of the upper gel was 120V, and the lower layer was The gel voltage was 180V, and the electrophoresis was ended when the bromophenol blue indicator band moved to the bottom of the gel, and then stained. The results of electrophoresis were as figure 2 As shown in -B, the electr...
Embodiment 3
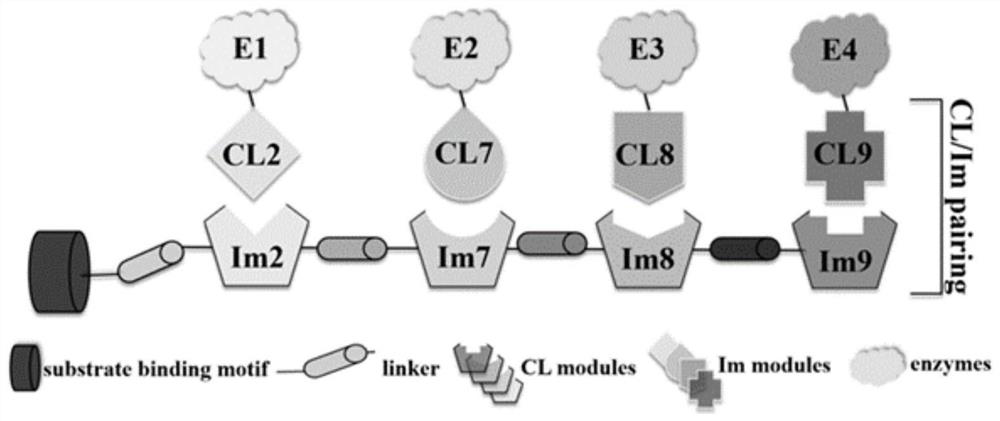
[0059] Example 3 Construction and thermal stability verification of scaffold protein Scaf-CIQ
[0060] 1. In this example, based on the above-mentioned combination of CL and Im protein, an interaction pair can be formed, and the corresponding scaffold protein is constructed, as follows:
[0061] The scaffold protein Scaf-CIQ in the CIQ system is a cellulose-binding module CBM3a derived from Clostridium thermocellum (living environment 60-90°C), and is a fusion protein expressed in tandem with Im2, Im7, Im8, and Im9 through a linker, which is the scaffold Protein Scaf-CIQ. The presence of CBM3a endows the scaffold protein Scaf-CIQ with the ability to bind to cellulose matrices such as microcrystalline cellulose (Avicel), phosphoric acid swollen cellulose (PASC), and achieve the purpose of targeting cellulose matrices and immobilizing enzymes. The principle of the scaffold protein Scaf-CIQ is as follows image 3 As shown, each Im module on the scaffold protein Scaf-CIQ can onl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com