Catalyst for preparing dinitrile compound from alicyclic hydrocarbon and synthetic method of dinitrile
A catalyst, alicyclic hydrocarbon technology, applied in the field of catalytic organic synthesis, can solve the problems of catalyst activity and selectivity to be improved, difficult activation of reaction raw materials, etc., to achieve the effects of easy process control, long reaction route and good selectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
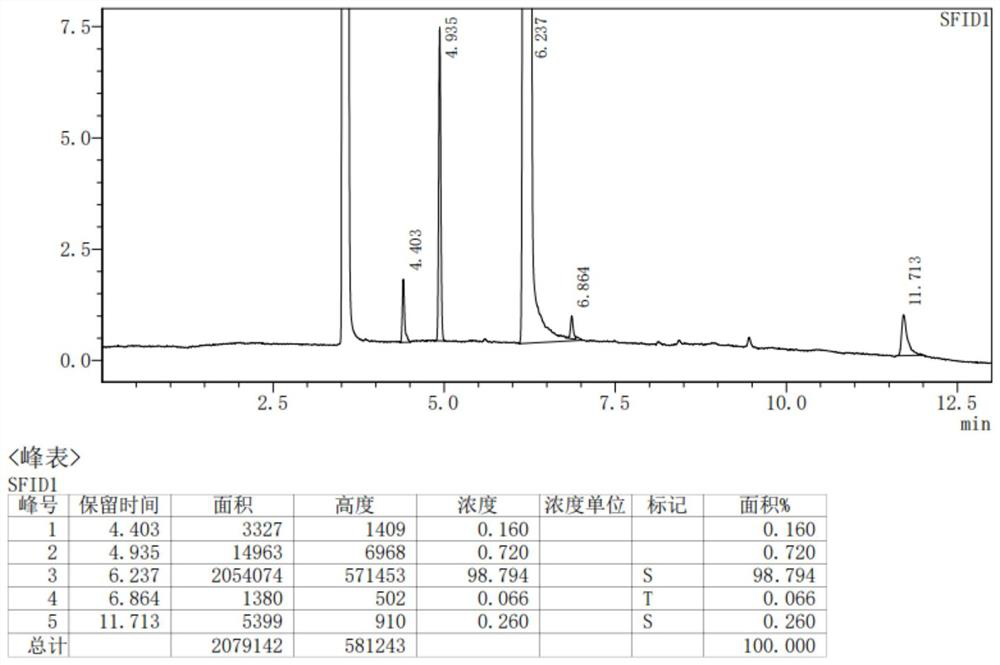
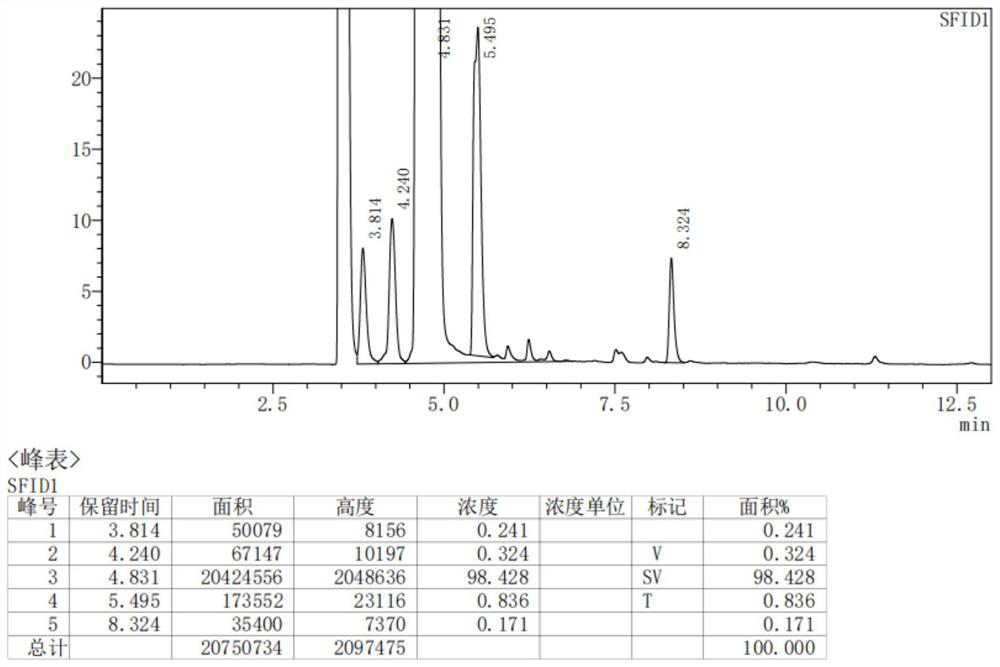
Image
Examples
Embodiment 1
[0093] 48 g of vanadium pentoxide was added to 800 g of oxalic acid solution with a mass concentration of 22.5 wt %, stirred for 2 hours, and dissolved to obtain a mixed solution. Add successively 35g titanium dioxide, 12.8g ammonium orthomolybdate ((NH 4 ) 2 MoO 4 ), 56g concentration is the phosphoric acid solution of 85.54wt%, stirs 2 hours, then slowly adds the nickel chloride NiCl that technical grade contains water of crystallization 2 ·6H 2 O 9.80g, 3.2g sodium chloride, stirred for 1 hour, then slowly added SnCl 2 2H 2 O 7.60 g and 10.5 g cesium sulfate, stirred for 1 hour, and then added 980 g of silica sol (Shandong Haoyao, silica hydrosol s40 type) with a mass concentration of 40 wt%, to obtain a catalyst slurry. The catalyst slurry was heated to evaporate water until the solid content of the system was 45%. The slurry is spray-dried in a spray dryer to obtain a catalyst precursor. The molar ratio of each metal element added is V:Ti:P:Mo:Cs:Na:Ni:Sn=1:0.83:0....
Embodiment 2
[0098] 48 g of vanadium pentoxide was added to 800 g of oxalic acid solution with a mass concentration of 22.5 wt %, stirred for 2 hours, and dissolved to obtain a mixed solution. Add 30g titanium dioxide, 10.3g ammonium orthomolybdate ((NH 4 ) 2 MoO 4 ), 68.3g concentration is the phosphoric acid solution of 85.54wt%, stirs 2 hours, then slowly adds the nickel chloride NiCl that technical grade contains water of crystallization 2 ·6H 2 O 4.80g, stirred for 1 hour, then slowly added SnCl 2 2H 2 O 7.60g and 15.3g of cesium sulfate were stirred for 1 hour, and then 980g of silica sol with a mass concentration of 40wt% was added to obtain a catalyst slurry. The catalyst slurry was heated to evaporate water until the solid content of the system was 45%. The slurry is spray-dried in a spray dryer to obtain a catalyst precursor. The molar ratio of the added metal elements is V:Ti:P:Mo:Cs:Ni:Sn=1:0.71:1.12:0.10:0.16:0.04:0.06.
[0099] Place the catalyst precursor in a muffle...
Embodiment 3
[0103] 48 g of vanadium pentoxide was added to 800 g of oxalic acid solution with a mass concentration of 22.5 wt %, stirred for 2 hours, and dissolved to obtain a mixed solution. Add 42.2g titanium dioxide, 5.2g ammonium orthomolybdate ((NH 4 ) 2 MoO 4 ), 67.7g of phosphoric acid solution with a concentration of 85.54wt%, stirred for 2 hours, then slowly added 4.3g of potassium chloride, stirred for 1 hour, then slowly added SnCl 2 2H 2 O 7.60g and 7.6g cesium sulfate, stirred for 1 hour, then added 980g of silica sol with a mass concentration of 40wt%, to obtain a catalyst slurry. The catalyst slurry was heated to evaporate water until the solid content of the system was 45%. The slurry is spray-dried in a spray dryer to obtain a catalyst precursor. The molar ratio of the added metal elements is V:Ti:P:Mo:Cs:K:Sn=1:1.00:1.12:0.05:0.08:0.11:0.06.
[0104] Place the catalyst precursor in a muffle furnace at 300°C for 3 hours and then gradually raise the temperature to 65...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com