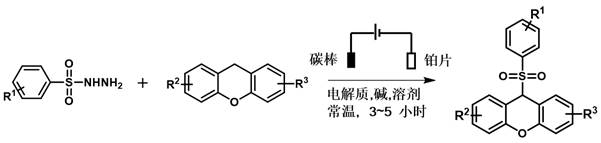
Method for electrochemical synthesis of alkyl sulfone compounds
A synthesis method and technology of alkyl sulfone, which is applied in the application field of alkyl sulfone compounds, can solve the problems of few reports, and achieve the effects of high atom economy, good inhibitory activity, and good functional group tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
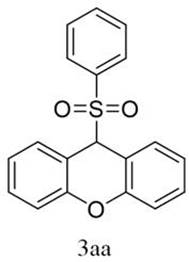
[0024] 9-(Benzenesulfonyl)-9 H - Synthesis and characterization of xanthene (3aa):
[0025]
[0026] 0.6 mmol benzenesulfonyl hydrazide, 0.3 mmol 9 H -Xanthene, 0.06 mmol ammonium iodide and 0.6 mmol potassium carbonate were added to a 25 mL three-necked flask, and 6 mL of a mixed solution of 1,2-dichloroethane and hexafluoroisopropanol (v:v = 4: 2) Dissolving, using a carbon rod as an anode and a platinum sheet as a cathode, stirring and reacting for 3 hours at a constant current of 10 mA at room temperature, and monitoring the reaction process by TLC;
[0027] After the reaction was complete, the mixture was extracted with ethyl acetate (10 mL). Anhydrous Na for organic layer 2 SO 4 Dry, spin the solvent under reduced pressure, and purify the residue by column chromatography (silica gel, petroleum ether / ethyl acetate = 1:20 elution) to obtain a white solid 3aa;
[0028] White solid (75%, 72.5 mg). mp: 193.8-194.6 o C, 1 H NMR (400 MHz, CDCl 3 ) δ 7.57-7.49 (m, 3H)...
Embodiment 2
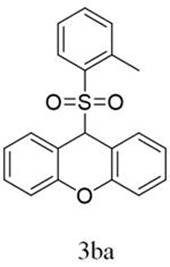
[0030] 9-(o-toluenesulfonyl)-9 H - Synthesis and characterization of xanthene (3ba):
[0031]
[0032] 0.6 mmol 2-methylbenzenesulfonyl hydrazide, 0.3 mmol 9 H -Xanthene, 0.06 mmol potassium iodide and 0.6 mmol cesium carbonate were added to a 25 mL three-necked flask, and 6 mL of a mixture of 1,2-dichloroethane and hexafluoroisopropanol (v:v = 4:2) was added Dissolve, use a carbon rod as an anode, and a platinum sheet as a cathode, carry out a stirring reaction for 4 hours at a constant current of 12 mA at room temperature, and monitor the reaction process by TLC;
[0033] After the reaction was complete, the mixture was extracted with ethyl acetate (10 mL). Anhydrous Na for organic layer 2 SO 4 Dry, spin the solvent under reduced pressure, and purify the residue by column chromatography (silica gel, petroleum ether / ethyl acetate = 1:30 elution) to obtain a white solid 3ba;
[0034] White solid (44%, 44.4 mg). mp: 216.7-217.8 o C, 1 H NMR (600 MHz, CDCl 3 ) δ 7.57-...
Embodiment 3
[0036] 9-(m-toluenesulfonyl)-9 H - Synthesis and characterization of xanthene (3ca):
[0037]
[0038] 0.6 mmol 3-methylbenzenesulfonyl hydrazide, 0.3 mmol 9 H -Xanthene, 0.06 mmol tetrabutylammonium iodide and 0.6 mmol sodium methoxide were added to a 25 mL three-necked flask, and 6 mL of a mixture of 1,2-dichloroethane and hexafluoroisopropanol (v:v = 4:2) dissolving, using a carbon rod as an anode, and a platinum sheet as a cathode, at a constant current of 15 mA, stirring and reacting for 5 hours under normal temperature conditions, TLC monitoring reaction process;
[0039] After the reaction was complete, the mixture was extracted with ethyl acetate (10 mL). Anhydrous Na for organic layer 2 SO 4After drying, the solvent was spin-dried under reduced pressure, and the residue was purified by column chromatography (silica gel, petroleum ether / ethyl acetate = 1:40 elution) to obtain a white solid 3ca;
[0040] White solid (70%, 70.6 mg). mp: 169.5-170.2 o C, 1 H NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com