Novel 4-butylresorcinol synthesis method
A technology of butyl resorcinol and butyryl resorcinol, which is applied in the field of synthesis of 4-butyl resorcinol, can solve the problems of easy safety accidents, high risk factor, a large amount of waste acid and mercury-containing solids. Waste and other problems, to avoid high-pressure dangerous reaction, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of 4-butyrylresorcinol
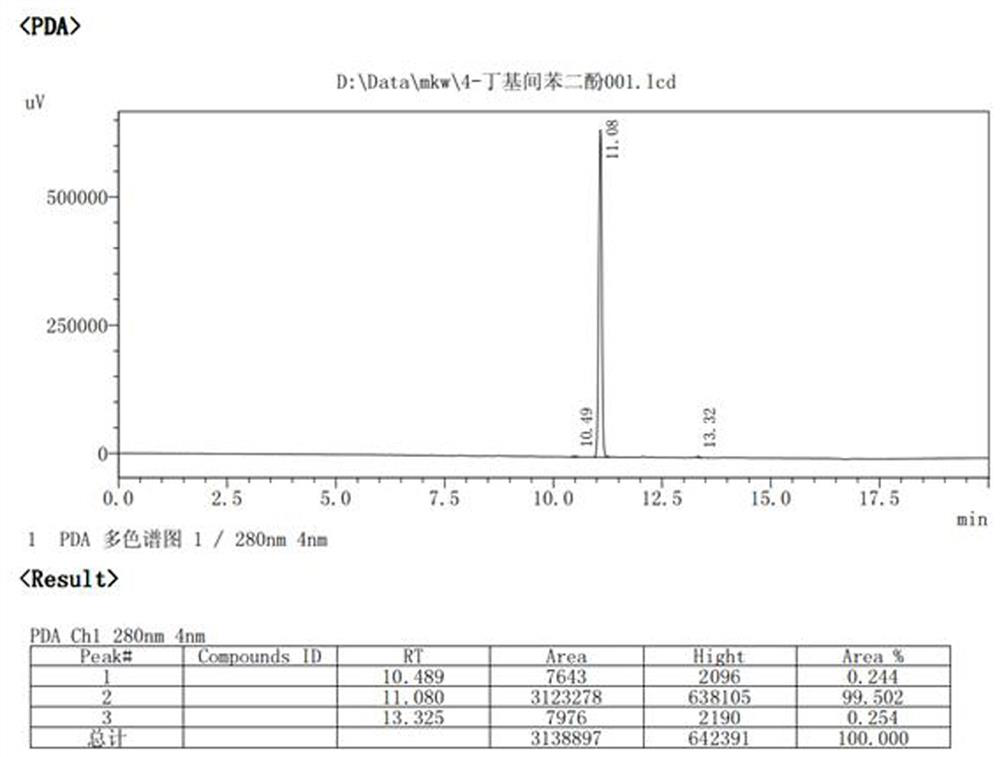
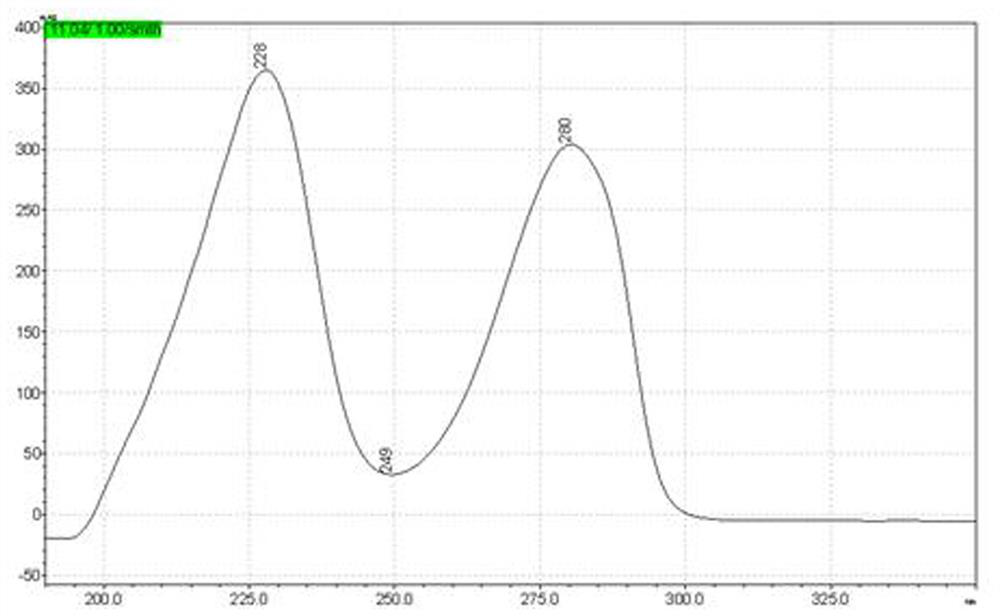
[0040] Add resorcinol (220g, 1.0 eq), toluene (700 mL), zinc chloride (350 g, 1.3 eq), butyric acid (220 g, 1.25 eq) into a 2000 mL three-necked flask, connect to the water separator, and heat up to 105-110°C heat preservation reaction for 4-6 hours, HPLC central control, after the reaction, add 500g of water to the reaction bottle to obtain a red solution, heat up and distill toluene until no more drops out, then cool down to room temperature, each time Extracted 2-3 times with 200ml of ethyl acetate, combined the organic phases, concentrated to dryness, the obtained oil was recrystallized with ethanol water, and dried to obtain the target intermediate product with a weight of 305g and a yield of 84.7%.
[0041] Synthesis of 4-Butylresorcinol
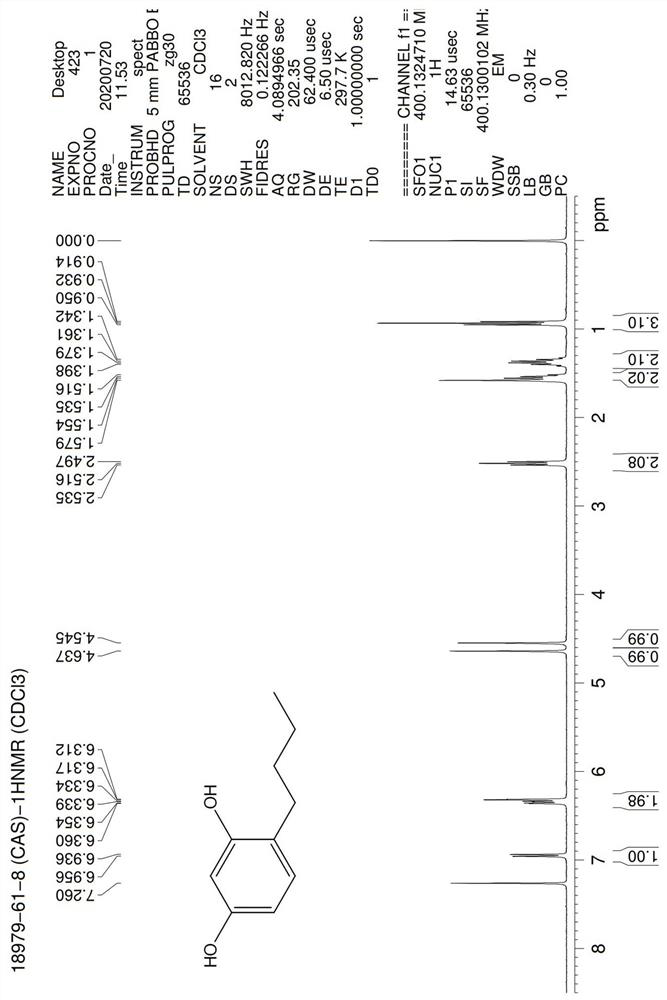
[0042] In a 100 mL three-neck flask, add 4-butyrylresorcinol (10 g, 1.0eq), 5% Pd / C (0.1g, 0.01X), methanol (40 ml, 4V), PMHS (10 mL , the hydrogen content is not less than 1.5%, 4-5eq hydrog...
Embodiment 2
[0044] The synthesis steps of 4-butyryl resorcinol in this example are the same as those in Example 1.
[0045] The difference between this embodiment and embodiment 1 is: the synthesis of 4-butylresorcinol
[0046] In a 100 mL three-neck flask, add 4-butyrylresorcinol (10 g, 1.0eq), 5% Pd / C (0.05g, 0.005X), ethanol (30 ml, 3V), PMHS ((10 mL, the hydrogen content is not less than 1.5%, 4-5eq hydrogen equivalent), heated to 60°C and stirred for 6 hours, the reaction in the HPLC control was completed, filtered palladium carbon, and then concentrated to dryness with an oil pump under reduced pressure, PE / EA (1: 0~10:1) was purified by column chromatography as the eluent to obtain 7 g of the product, the HPLC purity was 98.6%, and the yield was 75.6%.
Embodiment 3
[0048] The synthesis steps of 4-butyryl resorcinol in this example are the same as those in Example 1.
[0049] The difference between this embodiment and embodiment 1 is: the synthesis of 4-butylresorcinol
[0050] In a 2000 mL three-neck flask, add 4-butyrylresorcinol (300 g, 1.0eq), 5% Pd / C (2g, 0.007X), methanol (1200 ml, 4V), PMHS ((320 mL , the hydrogen content is greater than 1.7%, the hydrogen equivalent is greater than 5 eq), the temperature is raised to 50°C and stirred for 3-4 hours, the reaction in the HPLC control is completed, the palladium carbon is removed by nitrogen pressure filtration, the mother liquor is first evaporated to methanol at normal pressure, and then decompressed with an oil pump Concentrate to dryness, use n-hexane as a solvent for the oily matter, decolorize and recrystallize with activated carbon to obtain 201 g of the product, the HPLC purity is 99.1%, and the yield is 72.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com