5-aminolevulinic acid synthetase mutant as well as host cell and application thereof
一种氨基乙酰丙酸、宿主细胞的技术,应用在5-氨基乙酰丙酸合成酶的突变体及其宿主细胞和应用领域,能够解决不多、没有用ALA生物合成、未研究突变酶热稳定性等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0128] Embodiment 1. Construction of ALA synthetase mutation carrier
[0129] Utilize the Stratagene Series XL-II site-directed mutagenesis kit, designed 19 pairs of primers (see Table 1), using the pET21a-hemA wild-type plasmid as a template (refer to Zhang et al.Biotechnology Letters, 2013,35(5):763-768 for the construction process), using The above primers were amplified by PCR, and the 15th amino acid residue of HemA was mutated into lysine (K) and arginine (R), and the 29th amino acid residue was mutated into glutamine (Q) and arginine respectively. acid (R), tyrosine (Y), lysine (K), the 39th amino acid residue is mutated to tyrosine (Y), phenylalanine (F), arginine (R), the Amino acid residue 176 is mutated to asparagine (N), lysine (K), aspartic acid (D), and amino acid residue 206 is mutated to tyrosine (Y) and phenylalanine (F ), the amino acid residue at position 236 was mutated to aspartic acid (D), asparagine (N), and arginine (R), the amino acid residue at pos...
Embodiment 2
[0133] Example 2. Expression of ALA synthase mutant enzyme and detection of enzymatic properties
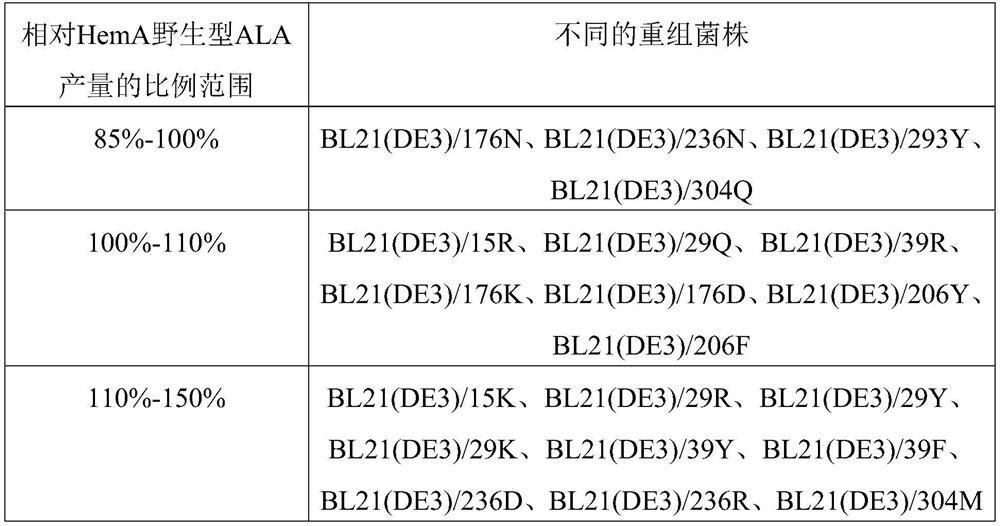
[0134] The expression vectors constructed in Example 1 were transformed into E.coli BL21(DE3) to obtain recombinant strains BL21(DE3) / pET21a-hemA, BL21(DE3) / pET21a-15K, BL21(DE3) / pET21a-15R, BL21(DE3) / pET21a-29Q, BL21(DE3) / pET21a-29R, BL21(DE3) / pET21a-29Y, BL21(DE3) / pET21a-29K, BL21(DE3) / pET21a-39Y, BL21(DE3) / pET21a-39F, BL21(DE3) / pET21a-39R, BL21(DE3) / pET21a-176N, BL21(DE3) / pET21a-176K, BL21(DE3) / pET21a-176D, BL21(DE3) / pET21a-206Y, BL21 (DE3) / pET21a-206F, BL21(DE3) / pET21a-236D, BL21(DE3) / pET21a-236N, BL21(DE3) / pET21a-236R, BL21(DE3) / pET21a-293Y, BL21(DE3) / pET21a -304M and BL21(DE3) / pET21a-304Q are used for the expression of different enzymes and the detection of enzymatic properties.
[0135]Inoculate 5 mL of LB liquid medium containing 100 μg / mL ampicillin with a single colony of the above-mentioned recombinant bacteria, and culture at 37° C. and 220 rpm for 12 hours. Trans...
Embodiment 3
[0146] Example 3. Application of ALA Synthetase Mutant Enzymes in ALA Synthesis
[0147] Inoculate 5 mL of LB liquid culture medium containing 100 μg / mL ampicillin with the preserved bacteria in glycerol tubes of the recombinant bacteria in Example 2, and culture them at 37° C. and 220 rpm for 12 hours. According to the initial OD of 0.05, transfer to a 250mL Erlenmeyer flask containing 30mL fermentation medium, culture at 37°C, 220rpm for 2.5h, then add IPTG with a final concentration of 0.05mM, induce culture for 9.5h, collect the fermentation broth, and detect the concentration of ALA. The shake flask fermentation medium formula is: glucose 15g / L, yeast powder 2.0g / L, Na 2 HPO 4 12H 2 O 17.1g / L, KH 2 PO 4 3.0g / L, NaCl 0.5g / L, NH 4 Cl 1.0g / L, MgSO 4 2.0mM, CaCl 2 0.1mM, glycine 4g / L, adjust the pH to 7.0. The final concentration of ampicillin was 100 μg / mL. ALA detection and glucose analysis methods are described in the "Materials and Methods" section. After det...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com