Penicillin modified epsilon-polylysine antibacterial peptide and preparation method thereof
A technology of polylysine and penicillin, which is applied in the direction of antibacterial drugs, antifungal agents, and medical preparations of non-active ingredients. It can solve the problems of limited antibacterial effect, complex synthesis, and rarely exert antibacterial properties. Enhanced performance, low manufacturing cost, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
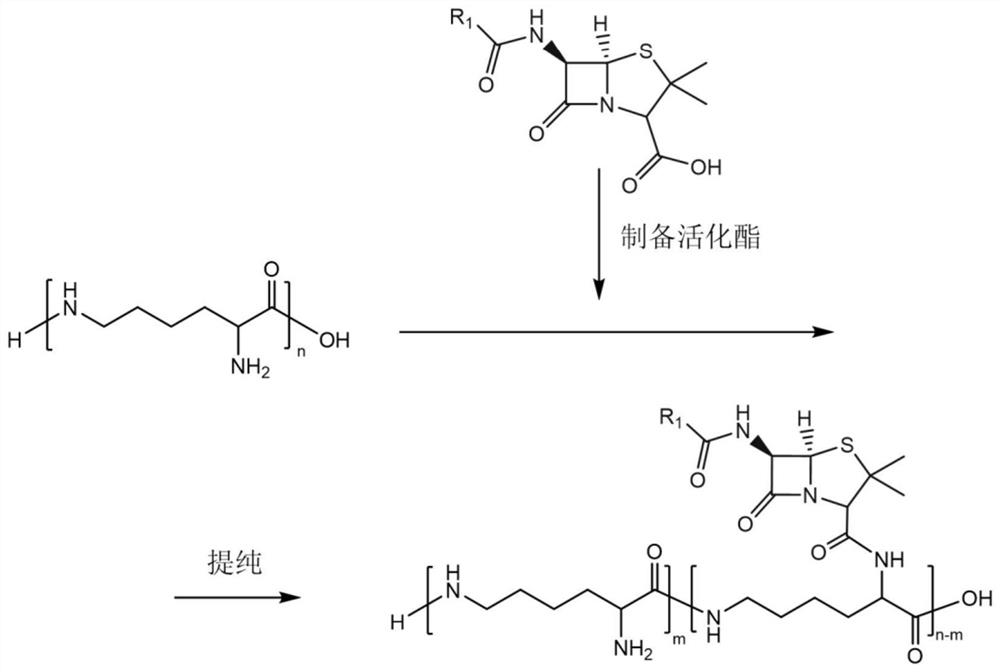
[0045] The preparation method of the epsilon-polylysine-Dipenicillin of the present embodiment comprises the following steps:
[0046] (1) Dissolve 3.72g (0.01mol) of penicillin potassium, 3.09g of N,N'-dicyclohexylcarbodiimide and 3.46g of N-hydroxysuccinimide in 15ml of pH=5 hydrochloric acid dilute solution and tetrahydrofuran mixed solvent (pH = 5 dilute hydrochloric acid solution: tetrahydrofuran = 2:1; v / v), react at 4°C for 3 hours to obtain penicillin activated ester monomer, as follows;
[0047]
[0048] (2) Add 5 mL of tetrahydrofuran to the activated penicillin ester to dissolve, add 30 mL of pre-dissolved deionized aqueous solution containing 16.115 g of ε-polylysine, and mix for 12 hours to obtain the ε-polylysine-dipenicillin.
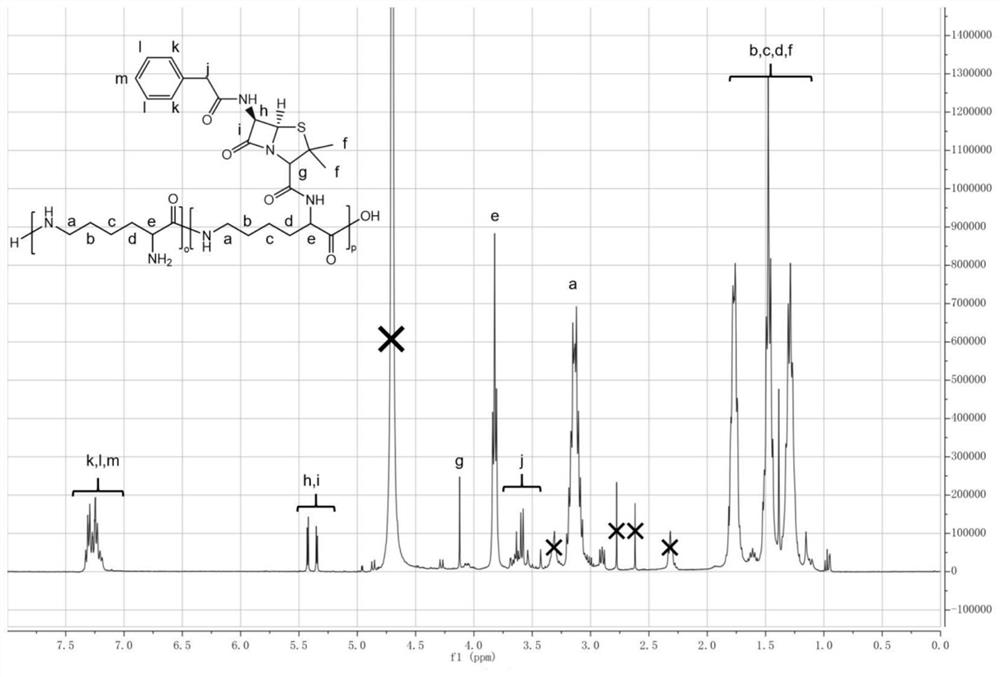
[0049] pass 1 H-NMR verifies the product structure, and the corresponding characteristic peaks of ε-polylysine-Dipenicillin can be obtained as figure 2 Shown: wherein the peak area ratio (66:5) of b-f and k-m is consistent with the ...
Embodiment 2
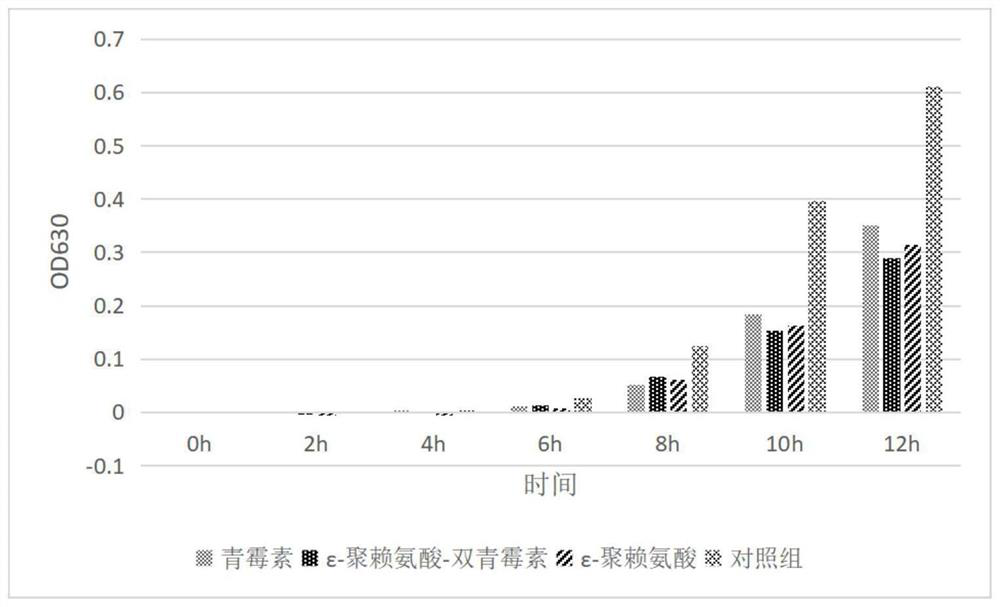
[0050] Embodiment 2: The determination of ε-polylysine-dipicillin minimum inhibitory concentration IC50 value
[0051] experimental method:
[0052] ε-polylysine-Dipenicillin source: Example 1
[0053] Determination by the micro-broth dilution method, firstly add the appropriate LB broth (sterilized by high pressure steam) into the 96-well plate, then the test sample (2mg / mL) obtained by lyophilization and the reference drug (penicillin, ε- Polylysine) solution (equal mass concentration) was added to the first well of each row of the 96-well plate, diluted by the double dilution method, and finally an equal volume of bacterial solution was inoculated into each well plate to obtain the concentration of each test group. The final drug concentrations were 500μg / mL, 250μg / mL, 125μg / mL, 62.5μg / mL, 31.25μg / mL, 16μg / mL, 8μg / mL. At the same time, two parallel blank control groups (that is, no sample added) were set up for the test, and incubated at a constant temperature of 37°C for 1...
Embodiment 3
[0056] Embodiment 3: Determination of hemolytic toxicity of ε-polylysine-Dipicillin
[0057] experimental method:
[0058] ε-polylysine-Dipenicillin source: Example 1
[0059] Adopt microdilution method to measure, at first configure the solution (2000 μ g / mL) of epsilon-polylysine-double penicillin and contrast drug (penicillin) with PBS buffer solution (pH=7.4), adopt double dilution method to half-dilution, then Add the same amount of blood cell PBS suspension to the sample solution to obtain the final drug concentrations of each test group as 1000 μg / mL, 500 μg / mL, 250 μg / mL, 125 μg / mL, 62.5 μg / mL, and use the same amount of PBS buffer And 0.1% Triton X-100 was added to the blood cell suspension to set up blank and positive control groups respectively. Incubate with repeated shaking for one hour. Centrifuge after culture, test the OD of each liquid supernatant 405 Value, and calculate the percentage of hemolysis in turn, the calculation formula is as follows:
[0060]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com