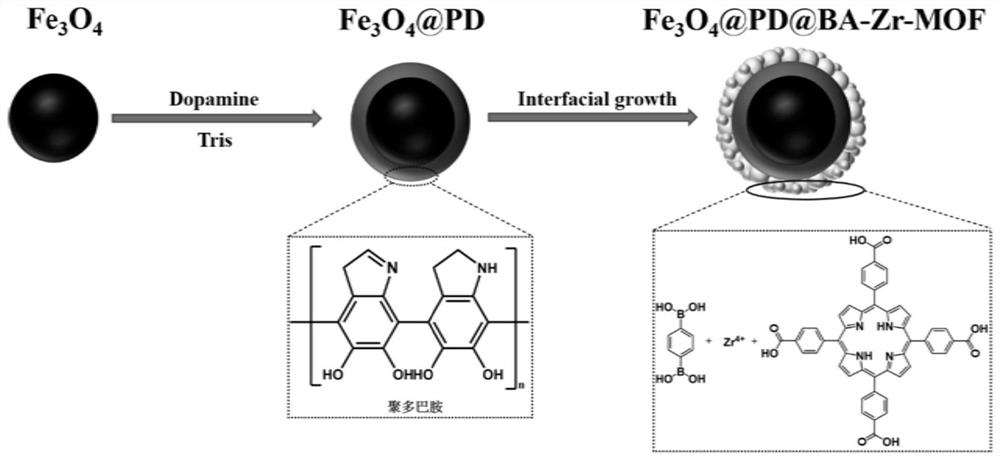
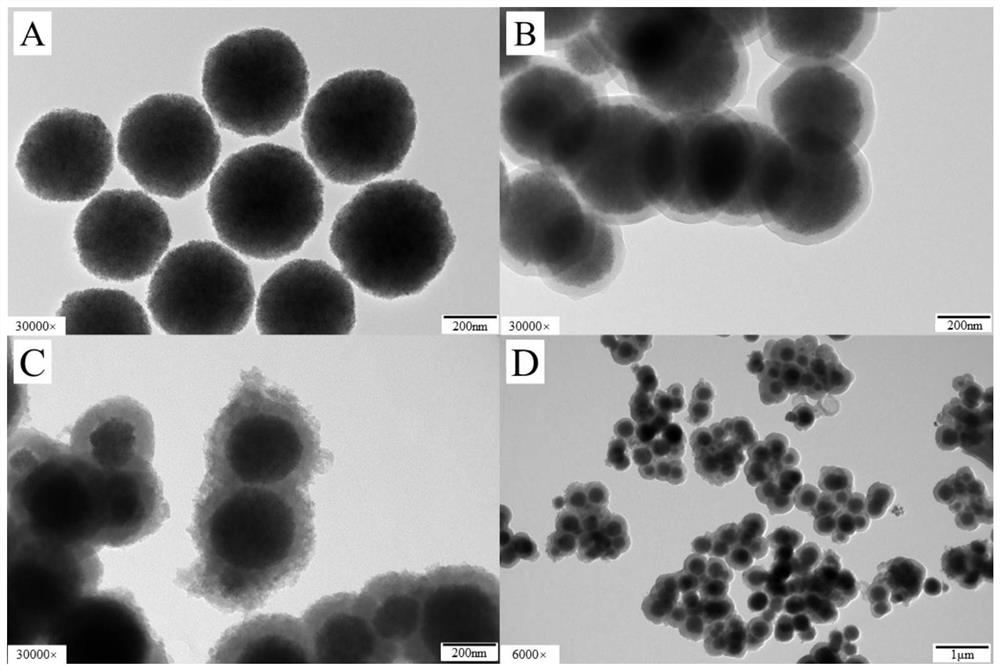
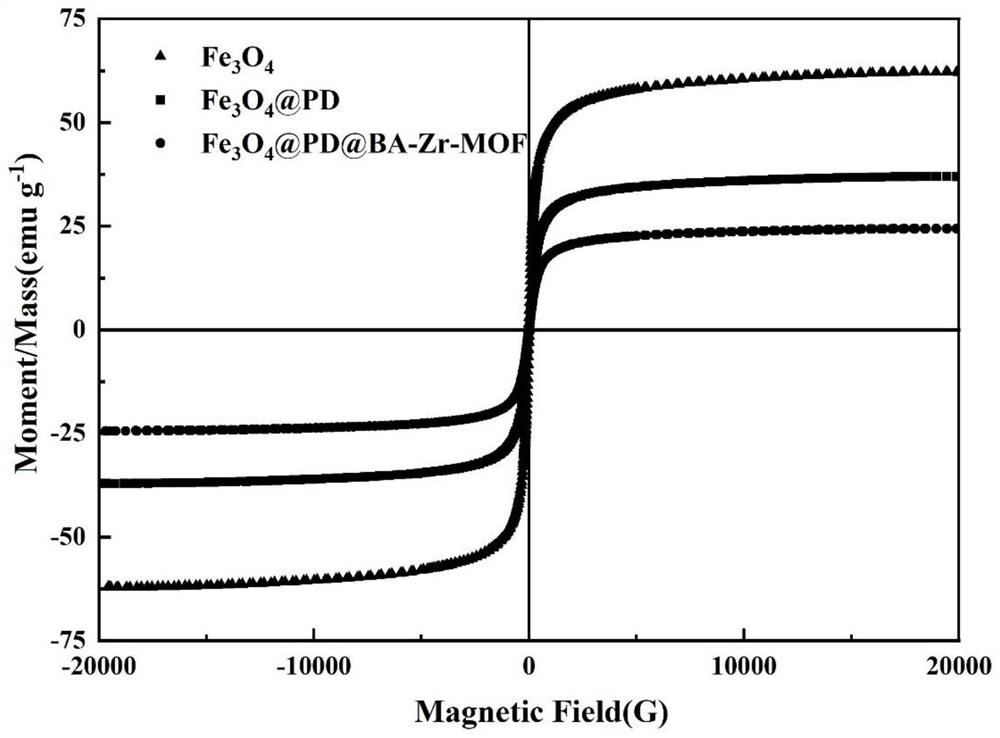
High-selectivity boric acid-doped metal organic framework magnetic adsorbent with core-shell structure as well as preparation method and application of high-selectivity boric acid-doped metal organic framework magnetic adsorbent
A metal-organic framework and magnetic adsorbent technology, applied in the field of analysis, can solve the problems of cumbersome enrichment operation, complicated preparation process, reduce non-specific adsorption, etc., achieve high enrichment capacity, simple operation steps, and reduce non-specific adsorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] This embodiment provides a method for preparing a metal-organic framework magnetic adsorbent doped with core-shell structure boric acid, comprising the following steps:
[0054] The first step: first use the hydrothermal method to synthesize Fe 3 o 4 Magnetic nanospheres: weigh 0.5g poly(4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA, molar ratio 3:1) and dissolve it in 20mL ethylene glycol under magnetic stirring, then add 0.54g FeCl 3 Dissolve 6H2O to obtain an orange-yellow clear solution, then add 1.5g of anhydrous sodium acetate, stir evenly, transfer the above solution to the reaction kettle, put it in an oven at 200°C for 10 hours, after the reaction is completed, cool to room temperature, and use a magnet The magnetic nanospheres were collected, washed several times with water and ethanol, and dried under vacuum to obtain brown Fe 3 o 4 magnetic nanospheres.
[0055] The second step: use polydopamine to modify magnetic nanospheres: prepare 120mL d...
Embodiment 2
[0061] This embodiment provides a method for preparing a metal-organic framework magnetic adsorbent doped with core-shell structure boric acid, comprising the following steps:
[0062] The first step: first use the hydrothermal method to synthesize Fe 3 o 4 Magnetic nanospheres: Weigh 0.1g poly(4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA, molar ratio 3:1) and dissolve it in 20mL diethylene glycol under magnetic stirring, then add 0.27g FeCl 3Dissolve 6H2O to obtain an orange-yellow clear solution, then add 1.5g of anhydrous sodium acetate, stir evenly, transfer the above solution to the reaction kettle, put it in an oven at 180°C for 20 hours, after the reaction is completed, cool to room temperature, and use a magnet The magnetic nanospheres were collected, washed several times with water and ethanol, and dried under vacuum to obtain brown Fe 3 o 4 magnetic nanospheres.
[0063] The second step: use polydopamine to modify magnetic nanospheres: prepare 120mL...
Embodiment 3
[0066] This embodiment provides a method for preparing a metal-organic framework magnetic adsorbent doped with core-shell structure boric acid, comprising the following steps:
[0067] The first step: first use the hydrothermal method to synthesize Fe 3 o 4 Magnetic nanospheres: Weigh 0.3g of poly(4-styrenesulfonic acid-co-maleic acid) sodium salt (PSSMA, molar ratio 3:1) and dissolve it in 20mL of ethylene glycol and diethylene glycol under magnetic stirring In the mixed solvent, add 1.08g FeCl 3 Dissolve 6H2O to obtain an orange-yellow clear solution, then add 1.5g of anhydrous sodium acetate, stir evenly, transfer the above solution to the reaction kettle, put it in an oven at 190°C for 5 hours, after the reaction is completed, cool to room temperature, and use a magnet The magnetic nanospheres were collected, washed several times with water and ethanol, and dried under vacuum to obtain brown Fe 3 o 4 magnetic nanospheres.
[0068] The second step: use polydopamine to ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com