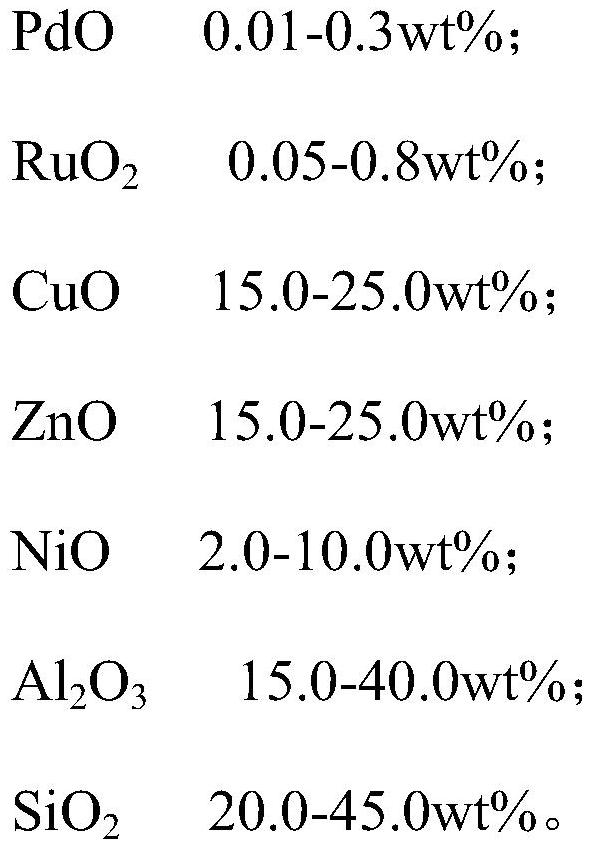
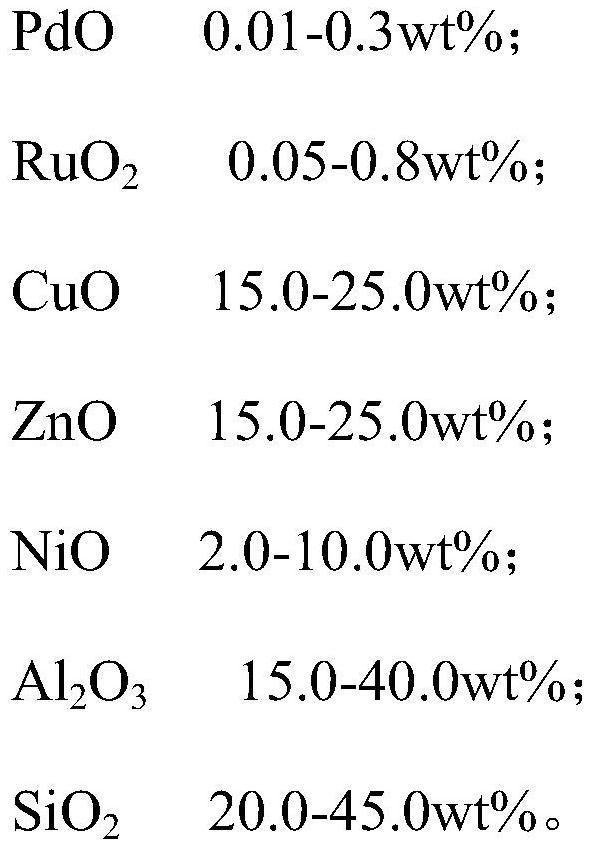
Dimethylbenzyl alcohol hydrogenolysis catalyst as well as preparation method and application thereof
A technology of dimethylbenzyl alcohol and catalyst, applied in the field of dimethylbenzyl alcohol hydrogenolysis catalyst and preparation thereof, can solve the problems of high catalyst cost, poor selectivity, poor liquid resistance and the like, and achieves excellent activity and selectivity, The catalyst pores are unobstructed and the liquid resistance is good.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] The present embodiment provides a preparation method of a dimethylbenzyl alcohol hydrogenolysis catalyst, and the preparation method comprises the following steps:
[0107] (1) Preparation of solution:
[0108] 672.4g of water was first added into the reaction kettle, then 96.4g of copper formate, 24.7g of nickel formate and 85.6g of zinc formate were added, and mixed solution 1 was obtained by fully stirring and dissolving;
[0109] Dissolve 133.3 g of urea in 755.2 g of water to obtain an aqueous urea solution, and then add 78.0 g of alkaline silica sol (SiO 2 content of 30wt%, particle size of 30nm, pH of 9.0) and fully stirred to obtain mixed solution 2;
[0110] Add 11.6g γ-aminopropyltrimethoxysilane to 253.5g nano-silica ethanol solution (SiO 2 content is 20wt%, particle size is 30nm), fully stirring to obtain mixed solution 3;
[0111] 0.5g of palladium acetylacetonate and 4.2g of ruthenium acetylacetonate were dissolved in 93.8g of benzene, and fully stirred...
Embodiment 2
[0122] The present embodiment provides a preparation method of a dimethylbenzyl alcohol hydrogenolysis catalyst, and the preparation method comprises the following steps:
[0123] (1) Preparation of solution:
[0124] 672.4g of water was added into the reactor, then 124.8g of copper formate, 12.4g of nickel formate and 75.0g of zinc formate were added, and mixed solution 1 was obtained by fully stirring and dissolving;
[0125] Dissolve 145.9 g of urea in 583.5 g of water to obtain an aqueous urea solution, and then add 83.6 g of alkaline silica sol (SiO 2 content of 40wt%, particle size of 40nm, pH of 9.0) and fully stirred to obtain mixed solution 2;
[0126] Add 6.5g γ-aminopropyltriethoxysilane to 264.0g nano-silica ethanol solution (SiO 2 content is 20wt%, particle size is 30nm), fully stirring to obtain mixed solution 3;
[0127] 0.75g of palladium acetylacetonate and 2.4g of ruthenium acetylacetonate were dissolved in 62.8g of benzene, and fully stirred to obtain mix...
Embodiment 3
[0135] The present embodiment provides a preparation method of a dimethylbenzyl alcohol hydrogenolysis catalyst, and the preparation method comprises the following steps:
[0136] (1) Preparation of solution:
[0137] 750.3g of water was first added into the reaction kettle, then 113.5g of copper formate, 39.6g of nickel formate and 78.2g of zinc formate were added, and mixed solution 1 was obtained by fully stirring and dissolving;
[0138] Dissolve 189.3 g of urea in 441.6 g of water to obtain an aqueous urea solution, and then add 70.0 g of alkaline silica sol (SiO 2 content of 30wt%, particle size of 30nm, pH of 9.0) and fully stirred to obtain mixed solution 2;
[0139] Add 6.8g aniline methyl trimethoxysilane to 248.0g nano-silica propanol solution (SiO 2 content is 15wt%, particle size is 30nm), fully stirring to obtain mixed solution 3;
[0140] 0.90g of palladium acetylacetonate and 1.2g of ruthenium acetylacetonate were dissolved in 41.9g of chloroform, and fully ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com