1, 2, 4-oxadiazole derivative containing 1, 3, 4-thiadiazole unit as well as preparation method and application of 1, 2, 4-oxadiazole derivative
A technology of oxadiazole and thiadiazole, which is applied in the fields of chemical industry and pesticides, can solve the problems of no nematicidal activity, low nematicidal activity in vitro, and lack of flexibility.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
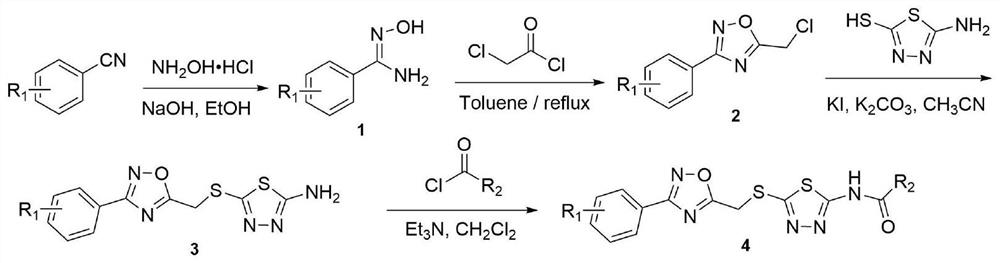
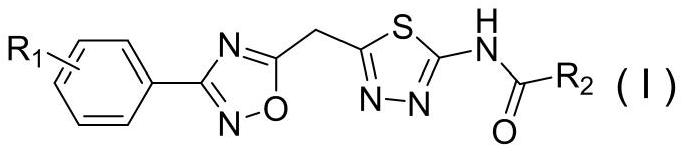
[0063] Example 1: 2-fluoro-N-(5-(((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)sulfur)-1,3,4-thiadiazole -2-yl) benzamide (i.e. compound 4a) preparation method, comprising the following steps:
[0064] (1) Preparation of N-hydroxybenzamide:
[0065] Hydroxylamine hydrochloride (4.04g, 58.18mmol), sodium hydroxide (2.33g, 58.18mmol), and ethanol (48.49mL) were mixed in a 100mL three-necked flask, and benzonitrile (5.00g, 48.49mmol) was added at room temperature, and heated after dropping Reflux for 7 hours; after the reaction, the reaction system was washed with water, separated, dried, suction filtered and desolvated under reduced pressure to obtain 5.50 g of N-hydroxybenzamide intermediate, with a yield of 83.31%;
[0066] (2) Preparation of 5-(chloromethyl)-3-phenyl-1,2,4-oxadiazole:
[0067] Chloroacetyl chloride (6.84g, 60.59mmol) was added dropwise into a mixed solution of N-hydroxybenzamide (5.50g, 40.40mmol) and 40.40mL of toluene under ice bath, and the temperature was rais...
Embodiment 2
[0072] Example 2: N-(5-((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4-thiadiazol-2-yl ) the preparation method of cyclopropanecarboxamide (being compound 4b), comprises the following steps:
[0073] Steps (1)~(3) are with embodiment 1;
[0074] (4) The target compound N-(5-((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4-thiadiazole-2- Base) the preparation of cyclopropanecarboxamide:
[0075] 5-((3-Phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4-thiadiazol-2-amine (0.20g, 0.69mmol) , triethylamine (0.17g, 1.72mmol) and 2.07mL of dry dichloromethane were added to the one-necked bottle, cyclopropylformyl chloride (86.11mg, 0.82mmol) was added dropwise into the system, and the reaction was carried out at room temperature for 6h; the solvent was removed under reduced pressure , recrystallized from methanol to give white solid N-(5-((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4-thiadiazole- 2-yl)cyclopropanecarboxamide 0.16g, yield 64.85%.
Embodiment 3
[0076] Example 3: 4-methoxy-N-(5-(((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4- The preparation method of thiadiazol-2-yl)benzamide (i.e. compound 4c), comprises the following steps:
[0077] Steps (1)~(3) are with embodiment 1;
[0078] (4) The target compound 4-methoxy-N-(5-(((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4 - Preparation of thiadiazol-2-yl)benzamide:
[0079] 5-((3-Phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1,3,4-thiadiazol-2-amine (0.20g, 0.69mmol) , triethylamine (0.17g, 1.72mmol) and 2.07mL of dry dichloromethane were added to the one-necked bottle, p-methoxybenzoyl chloride (0.14g, 0.82mmol) was added dropwise into the system, and the reaction was carried out at room temperature for 6h; The solvent was removed and recrystallized from methanol to obtain a white solid 4-methoxy-N-(5-(((3-phenyl-1,2,4-oxadiazol-5-yl)methyl)thio)-1 ,3,4-Thiadiazol-2-yl)benzamide 0.15g, the yield was 51.36%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com