Carboxyl functionalized super-crosslinked polymer as well as preparation method and application thereof
A technology of carboxyl functionalization and hypercrosslinking, which is applied in chemical instruments and methods, water pollutants, water/sludge/sewage treatment, etc., can solve the problems of unsatisfactory adsorption effect and selectivity, and increase the selective adsorption capacity , Ease of elution, and the effect of improving the selective adsorption capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1) Preparation of carboxylated polymer (PS-BTCA):
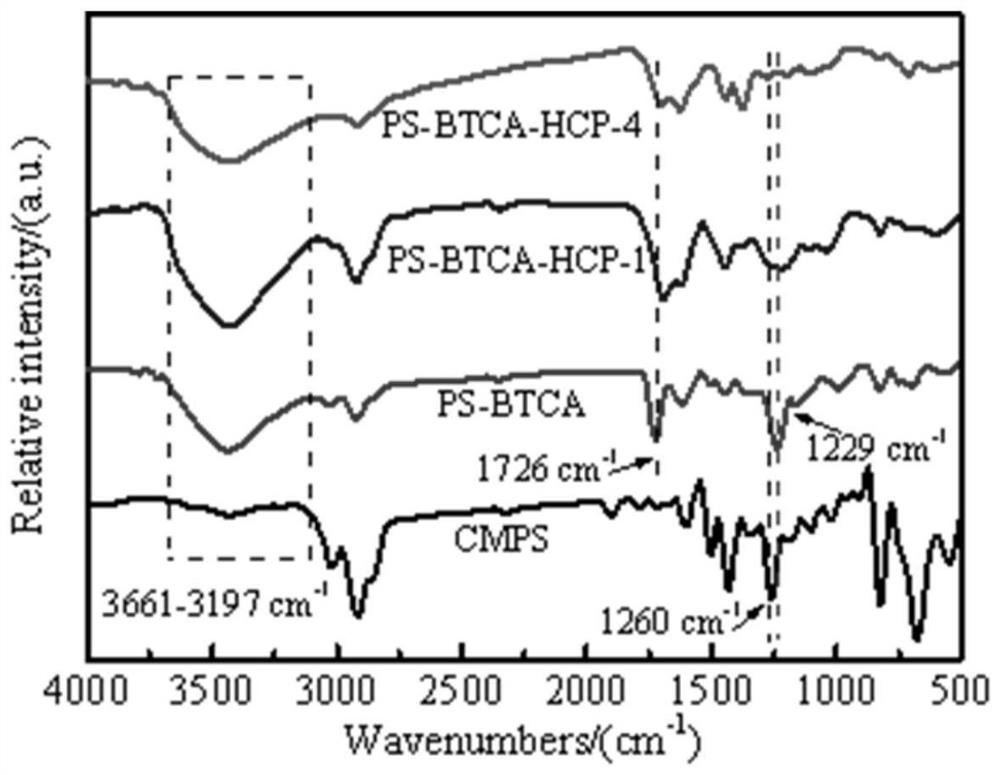
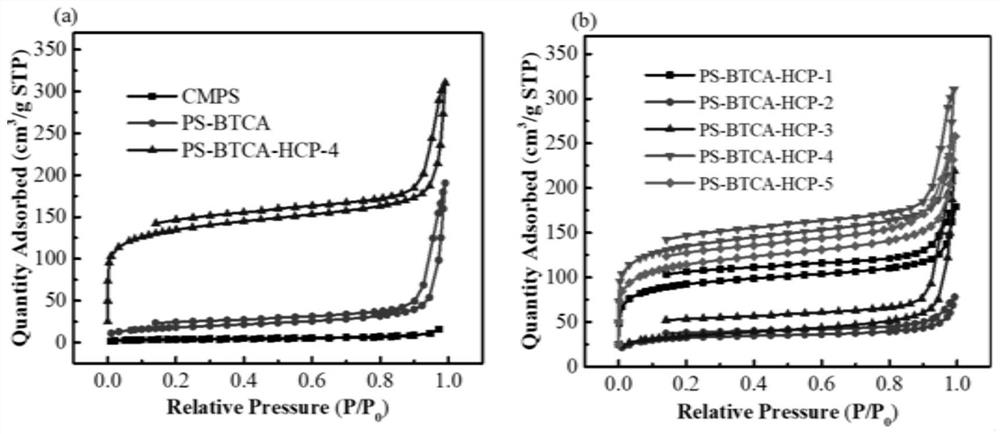
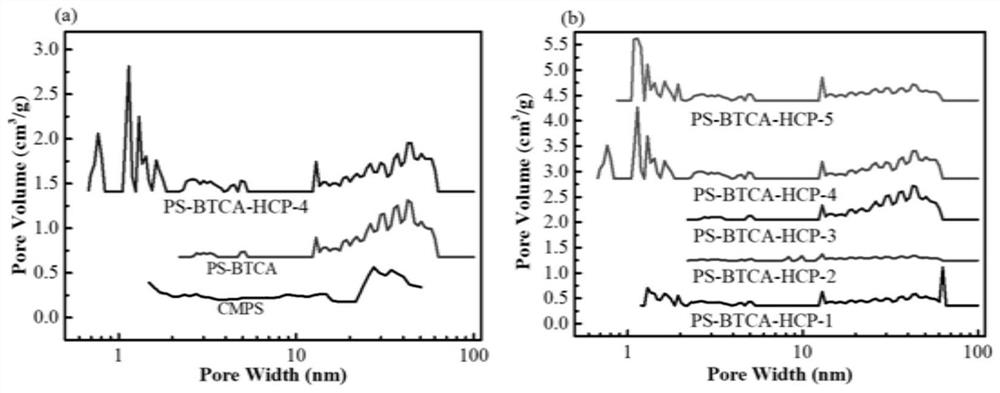
[0041] Add 10 g of macroporous low-crosslinked chloromethylated polystyrene microspheres (CMPS) to 100 mL of N,N-dimethylformamide (DMF), and allow the CMPS to fully swell at room temperature for 12 h. Add 8.524g trimesic acid and 20mL organic base N,N-diisopropylethylamine (the mol ratio of the chlorine content of CMPS, organic base, trimesic acid is 3:3:1), and the mixed reaction system is heated up to The reaction was continued at 90°C for 24h. The nucleophilic substitution reaction product was named PS-BTCA, and was washed repeatedly with deionized water, absolute ethanol, and 1% hydrochloric acid to remove residual organic bases and other substances. When the pH of the last washing liquid is neutral, stop the suction filtration, put the product into the Soxhlet extractor for extraction for 24 hours (the extraction solution should be clear and transparent), put the extracted product in the fume hood for about 1 ho...
Embodiment 2
[0057] 1) Preparation of carboxylated polymer (PS-PA):
[0058] Add 10 g of macroporous low-crosslinked chloromethylated polystyrene microspheres (CMPS) to 100 mL of N,N-dimethylformamide (DMF), and allow the CMPS to fully swell at room temperature for 12 h. Install a reflux condenser and a mechanical stirrer, add 4.04g phthalic acid and 4.8g basic catalyst K at room temperature 2 CO 3 (The molar ratio of the chlorine content of CMPS, the basic catalyst, and phthalic acid is 2:2:1), after stirring for 30 minutes until it is completely dissolved, the temperature is raised to 90° C., and the reaction is carried out at this temperature for 24 hours. Wash with 1% hydrochloric acid aqueous solution, ionized water, and absolute ethanol alternately for 3 to 4 times until colorless, then extract in a Soxhlet extractor overnight with ethanol, methanol, and water at a volume ratio of 1:1:1, and dry it normally for 12 hours. Then vacuum-dried for 24 hours to obtain the carboxylated pol...
Embodiment 3
[0065] 1) Preparation of carboxylated polymer (PS-BA):
[0066] Add 10 g of macroporous low-crosslinked chloromethylated polystyrene microspheres (CMPS) to 100 mL of N,N-dimethylformamide (DMF), and allow the CMPS to fully swell at room temperature for 12 h. Install reflux condenser and mechanical stirrer, add 6.14g benzoic acid and 4.8g basic catalyst K at normal temperature 2 CO 3(The molar ratio of the chlorine content of CMPS, the basic catalyst, and trimesic acid is 1:1:1), after stirring for 30 minutes until completely dissolved, the temperature is raised to 90° C., and the reaction is carried out at this temperature for 24 hours. Wash with 1% aqueous hydrochloric acid, ionized water, and absolute ethanol alternately for 3 to 4 times until colorless, then extract in a Soxhlet extractor overnight with ethanol, methanol, and water at a volume ratio of 1:1:1, and dry for 12 hours , and then vacuum-dried for 24h to obtain carboxylated polymer PS-BA;
[0067] 2) Preparatio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Pore volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com