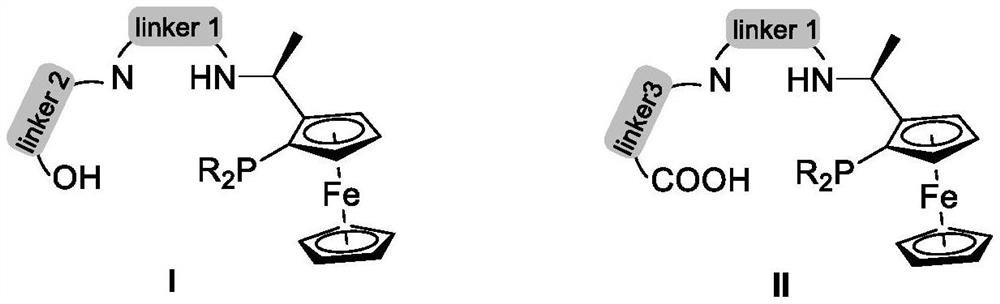
Chiral ferrocene PNNO tetradentate ligand and application thereof in asymmetric hydrogenation reaction
A technology of chiral ferrocene and tetradentate ligands, applied in the field of fine chemical industry, can solve the problem of less research on tetradentate ligands, and achieve the effects of excellent stereoselectivity, high yield and easy modification
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Example 1: Synthesis of Key Fragment 3 (Compound 3) (R=Ph)
[0065]
[0066] Under nitrogen atmosphere and 0°C, 15mL of n-BuLi n-hexane solution (2.4mol / L, 36mmol) was added dropwise to 7.7g of compound 1 (30mmol, 1.0equiv.) in anhydrous ether (60mL) solution, dropwise After the addition was completed, it was naturally raised to room temperature and stirred for 2.0h. Ph 2 PCl (33mmol, 1.2equiv.) was dropped into the reaction solution, heated to reflux, monitored by TLC, quenched with water after the reaction was completed, extracted with ether to obtain an organic phase, dried with anhydrous sodium sulfate, filtered, and spin-dried to obtain a red oil The liquid was recrystallized to obtain 10.3 g of the product compound 2 with a yield of 78%.
[0067] Under nitrogen protection, a mixture of compound 2 (2 mmol) and acetic anhydride (2 mL) was heated at 100° C. for about 1˜2 h. After the reaction was completed, the acetic anhydride was removed under reduced pressur...
Embodiment 2
[0068] Example 2: Synthesis of key fragment 3 (compound 3) (R=3,5-di-tert-butylphenyl)
[0069]
[0070] Under nitrogen atmosphere and 0°C, 5 mL of n-BuLi n-hexane solution (2.4mol / L, 12mmol) was added dropwise to a solution of compound 1 (2.57g, 10mmol) in anhydrous ether (20mL). After the addition was complete, Naturally rose to room temperature and stirred for 2h. Then the temperature was lowered to -78°C, and redistilled PCl was slowly added dropwise. 3 (12 mmol, 1 mL), the mixture was warmed to room temperature and reacted overnight. Then the temperature was lowered to -78°C again, and RMgBr solution (25 mmol, newly prepared) was slowly added dropwise with a constant pressure funnel. After the dropwise addition, slowly warm up to room temperature overnight, then add 20 mL of saturated NH 4 Cl solution. The organic phase was extracted three times with ether, 20 mL of ether each time. The organic phase was dried with anhydrous sodium sulfate, spin-dried, and subject...
Embodiment 3
[0072] Embodiment 3: the synthesis of key segment 3 (compound 3) (R=cyclohexyl)
[0073]
[0074] Under nitrogen atmosphere and 0°C, 5 mL n-BuLi n-hexane solution (2.4 mol / L, 12 mmol) was added dropwise to 2.57 g (S)-1 (10 mmol, 1.0 equiv.) solution in anhydrous ether (20 mL) After the dropwise addition, it was naturally raised to room temperature and stirred for 2.0h. Cy 2 PCl (33mmol, 1.2equiv.) was added dropwise to the reaction solution, heated to reflux, monitored by TLC, quenched with water after the reaction was completed, extracted with ether to obtain an organic phase, dried with anhydrous sodium sulfate, filtered, and spin-dried to obtain a red oil Liquid, recrystallized to obtain 2.17g of yellow solid compound 2, yield 50%.
[0075]Under nitrogen protection, a mixture of compound 2 (2 mmol) and acetic anhydride (2 mL) was heated at 100° C. for about 1˜2 h. After the reaction was completed, the acetic anhydride was removed under reduced pressure to obtain compo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com