Isopentenyl transferase mutant and method for producing cannabinoid phenol
A technology of isopentenyl and transferase, applied in the biological field, can solve the problem of low purity of catalytic activity and achieve the effect of high production and application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0157] Example 1: Construction of recombinant strains and expression process of mutants
[0158] (1) Construction of expression plasmids
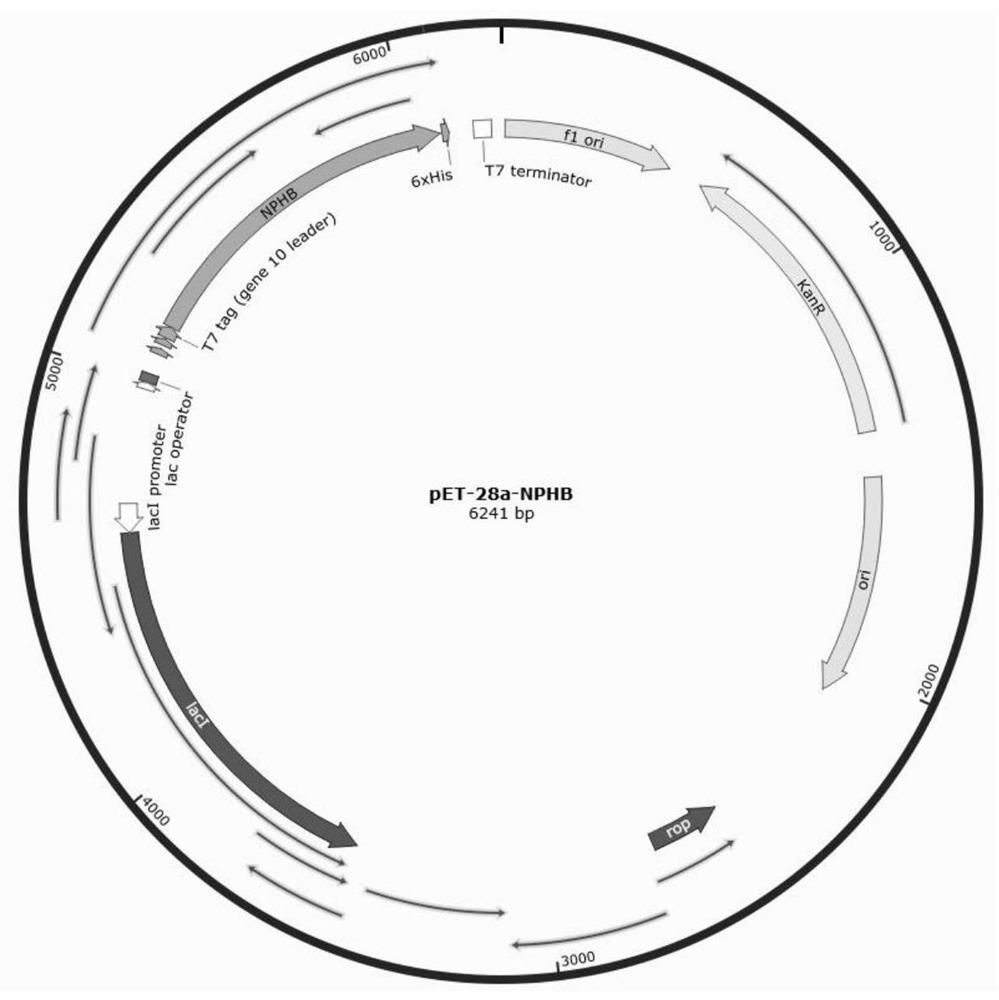
[0159] The NphB coding sequence derived from Streptomyces sp. species is codon-optimized according to the codon preference of Escherichia coli (E.coli), and then according to figure 1 Connected to the pet-28a plasmid (purchased from Beijing Suo Laibao Biotechnology Co., Ltd.), transformed into competent E. coli (purchased from Nanjing Novizan Biotechnology Co., Ltd.), cultured for 8h-12h, streaked and plated to prepare pET - Monoclonal strain of 28a-NphB.
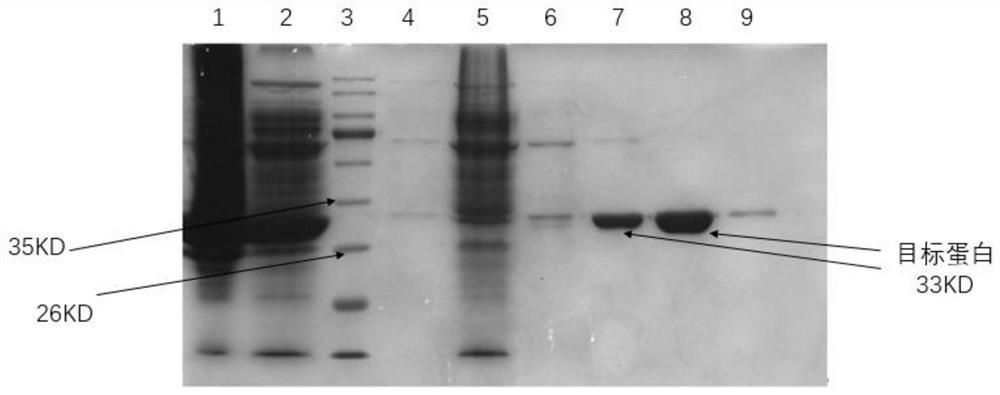
[0160] (2) Expression and purification of protein:
[0161] Prepare protein purification buffer: 50mM Tris, 150mM NaCl, PH=8
[0162] a. Pick the monoclonal strain of pET-28a-NphB or its strain stored at -80°C and inoculate it into a small test tube containing 5 mL of LB liquid medium (Kan+, 100 μg / mL), and culture it overnight at 37°C and 220 rpm as seeds liquid.
[0163] b. Transf...
Embodiment 2
[0182] Example 2: Olivol and GPP are catalyzed by protease NphB to generate CBG in vitro
[0183] This example aims to utilize the wild-type and M1-M19 mutants produced in Example 1 to catalyze the production of CBG from olivetol and GPP in vitro.
[0184] The way it is synthesized is as follows:
[0185]
[0186] Reaction buffer: 50 mM Tris-HCl, pH=8.0.
[0187] MgCl 2 The final concentration was 5 mM.
[0188] The final concentration of GPP was 2.5 mM.
[0189] The final concentration of Olivetol: 5mM.
[0190] The amount of NphB protease is 50ng (that is, the amount of NphB protease added is 1ng / uL).
[0191] The reaction temperature was 24°C.
[0192] Reaction time: 12h.
[0193] The total reaction system is: 50uL.
[0194] After the reaction was extracted twice with ethyl acetate, the solution was evaporated to dryness by a vacuum rotary evaporator, and finally dissolved with 50 uL of methanol to obtain a product containing CBG.
Embodiment 3
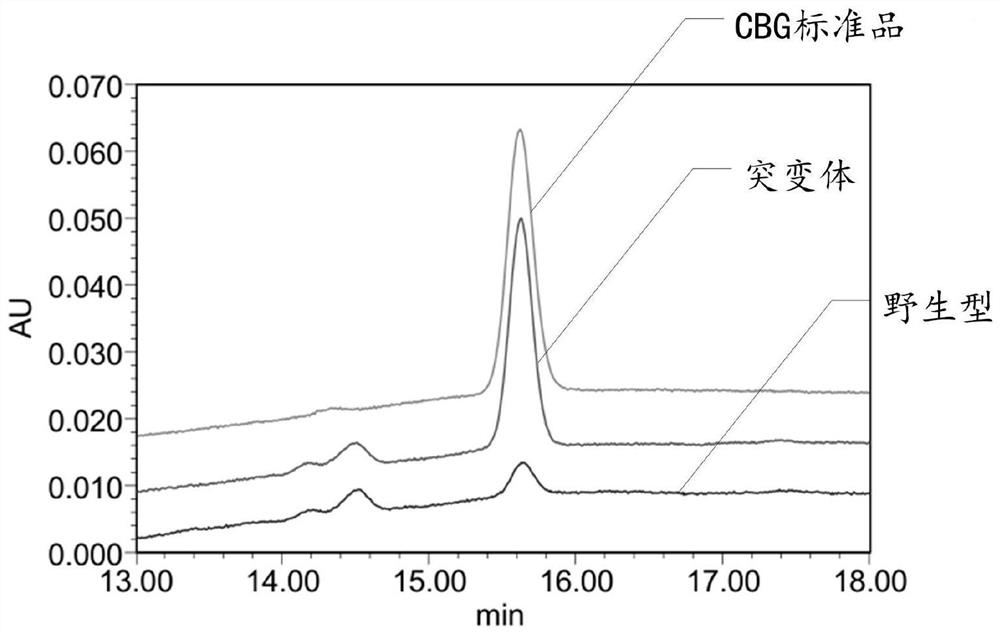
[0195] Embodiment 3: The detection of liquid phase and mass spectrometry is carried out to the CBG that reaction generates
[0196] The purpose of this example is to identify the product generated in Example 2.
[0197] Experimental equipment: ultra-high performance liquid chromatography-mass spectrometry (Shimadzu LC-30A, SCIEX TripleTOF6600).
[0198] Chromatographic conditions: flow rate: 0.4mL / min; column temperature 30°C; chromatographic column: Agilent Eclipse plus C18 (100mm×2.1mm, 3.5μm); mobile phase A: 5mM ammonium bicarbonate aqueous solution; mobile phase B: 0.005% formic acid acetonitrile Solution; Gradient: 0min~2min: 1%B, 2min~2.1min: 1~10%B, 2.1min~12min: 10~80%B, 12min~12.1min: 80~100%B, 12.1min~20min: 100B%.
[0199] Mass spectrometry conditions: ESI ion source; negative ion IDA detection mode; ion source parameters: ion voltage 4500 V, declustering potential 80 V, source temperature 600 ℃, curtain gas 35 psi, nebulizer gas 55 psi, heater gas 55 psi; primar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com