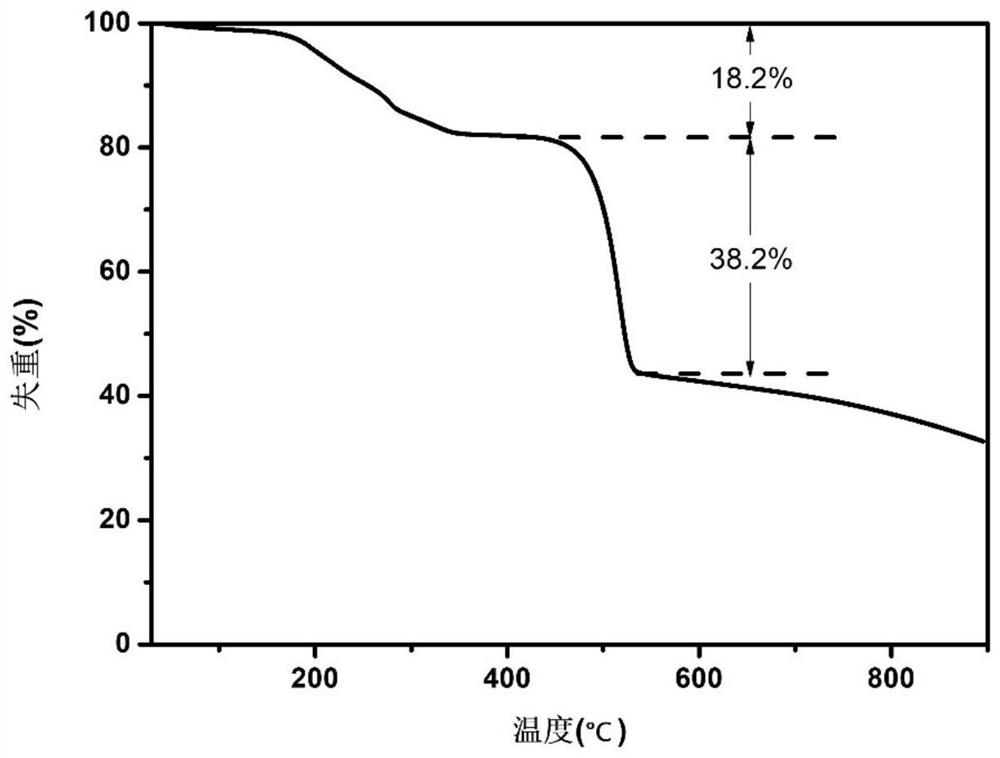
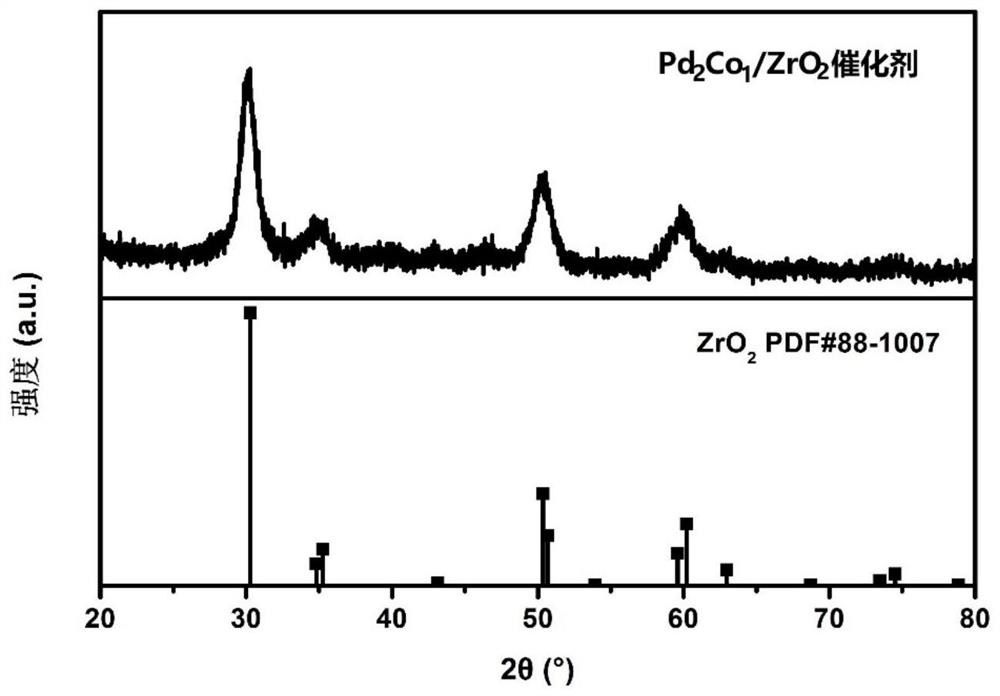
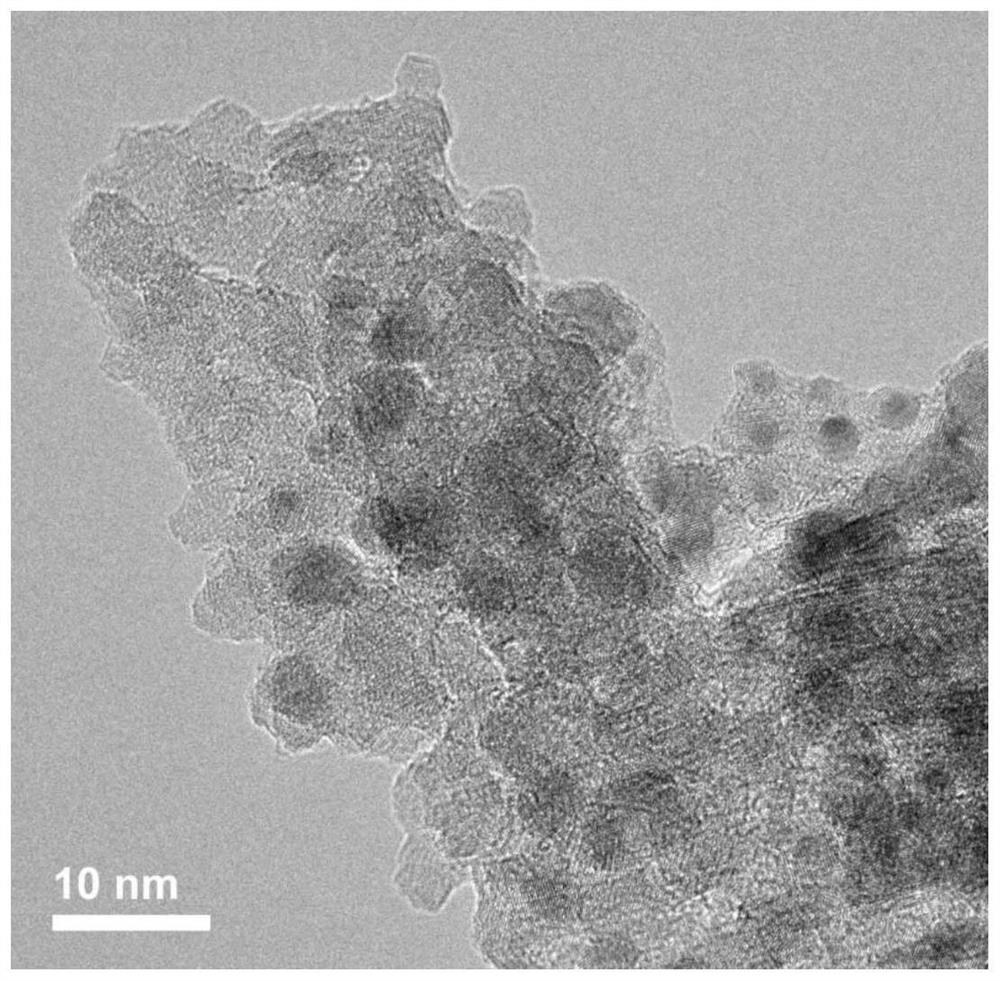
Solid acid-bimetallic nanoparticle composite material as well as preparation method and application thereof
A bimetallic nano-composite material technology, applied in the field of solid acid-bimetallic nanoparticle composite material and its preparation, can solve the problems of easy carbon deposition and deactivation, polychlorinated by-products, low reactivity, etc., to improve the oxidation reaction Activity, efficient catalytic conversion, enhanced interaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] A solid acid-bimetallic nanoparticle composite material, comprising the following steps:
[0073] 1) Preparation of UiO-67-H immobilized PdCo salt material
[0074] Dissolve 1 mmol of zirconium chloride, 1 mmol of 2,2'-bipyridyl-5,5'-dicarboxylic acid, and 1 mmol of glacial acetic acid in 60 mL of N,N-dimethylformamide, and react at 120°C for 24 hours to obtain The solid product was filtered, washed, and dried under vacuum at 150°C for 12 hours to obtain the acidified metal-organic framework support material UiO-67-H.
[0075] A certain amount of bisacetonitrile, palladium chloride and cobalt nitrate were fully dissolved in 30mL of acetonitrile, and then acidified metal organic framework support material UiO-67-H was added to control the Pd 2+ / Co 2+ The molar ratio is 2:1, while controlling the total metal content (Pd 2+ +Co 2+ ) is 1wt% of the carrier UiO-67-H, react at 65°C for 24h, filter and wash after cooling to room temperature, and vacuum dry at 150°C for 12...
Embodiment 2
[0091] A solid acid-bimetallic nanoparticle composite material, comprising the following steps:
[0092] 1) Preparation of UiO-67-H immobilized PdCo salt material
[0093] Dissolve 1 mmol of zirconium chloride, 1 mmol of 2,2'-bipyridine-5,5'-dicarboxylic acid, and 1 mmol of hydrochloric acid in 60 mL of N,N-dimethylformamide, and react at 120°C for 24 hours to obtain The solid product was filtered, washed, and dried under vacuum at 150°C for 12 hours to obtain the acidified metal-organic framework support material UiO-67-H.
[0094] Dissolve a certain amount of bisacetonitrile palladium chloride and cobalt acetate in 30mL of acetonitrile, then add the acidified metal organic framework support material UiO-67-H, control the Pd 2+ / Co 2+ The molar ratio is 2:1, while controlling the total metal content (Pd 2+ +Co 2+ ) is 0.25wt% of the carrier UiO-67-H, reacted at 65°C for 24h, filtered and washed after cooling to room temperature, and vacuum dried at 150°C for 12h to obtain...
Embodiment 3
[0099] A solid acid-bimetallic nanoparticle composite material, comprising the following steps:
[0100] 1) Preparation of UiO-67-H immobilized PdCo salt material
[0101] Dissolve 1 mmol of zirconium chloride, 1 mmol of 2,2'-bipyridine-5,5'-dicarboxylic acid, and 1 mmol of hydrochloric acid in 60 mL of N,N-dimethylformamide, and react at 120°C for 24 hours to obtain The solid product was filtered, washed, and dried under vacuum at 150°C for 12 hours to obtain the acidified metal-organic framework support material UiO-67-H.
[0102] Fully dissolve a certain amount of palladium nitrate and cobalt chloride in 30mL of acetonitrile, then add the acidified metal organic framework support material UiO-67-H, control the Pd 2+ / Co 2+ The molar ratio is 2:1, while controlling the total metal content (Pd 2+ +Co 2+ ) is 2.5wt% of the carrier UiO-67-H. React at 65°C for 24h, cool to room temperature, filter and wash, and vacuum dry at 150°C for 12h to obtain UiO-67-H immobilized PdCo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com