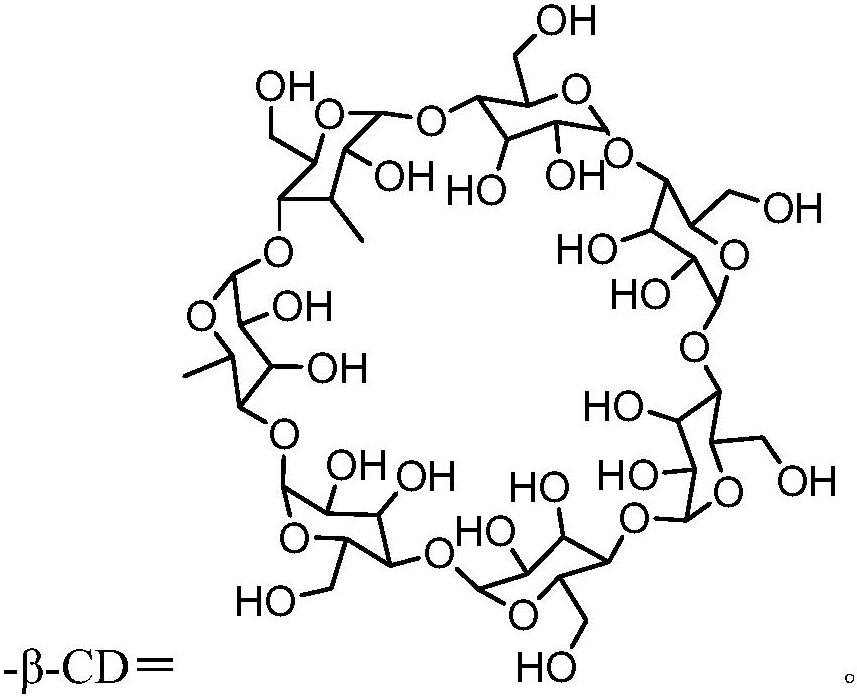
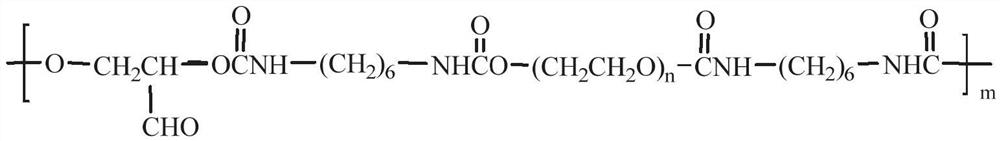
Polyurethane, single-component light-sensitive reversible hydrogel as well as preparation method and application of single-component light-sensitive reversible hydrogel
A technology of polyurethane and hydrogel, which is applied in the direction of non-active components of polymer compounds, aerosol delivery, emulsion delivery, etc. It can solve the problems of insufficient sensitivity of medium light sensitivity, insufficient sensitivity of light-sensitive groups, and difficult control of mutation range, etc. , to achieve excellent photoresponse speed, easy to control the content of functional groups, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Preparation of PU-CD-AB:
[0054] Dissolve 50g of polyethylene glycol, 4.5g of 2,3-dihydroxypropionaldehyde and 0.2g of dibutyltin dilaurate in 72mL of DMF, stir well, add 10.3g of hexamethylene diisocyanate, and mechanically stir , reacted at 80°C for 3.0h until the infrared characteristic absorption peak of -NCO disappeared. 5 times the volume of glacial ether was settled, and vacuum-dried to obtain polyurethane PU-AG-I containing aldehyde groups in the side chain (the weight-average molecular weight was 5.6×10 4 g / mol);
[0055] Dissolve 10g of PU-AG-I in 20mL of DMF, cool down to 15°C, and add monoamino-β-cyclodextrin (β-CD-NH 2 ) DMF solution (4.37g is dissolved in 4.5mLDMF), maintain the temperature reaction for 1.5, continue to dropwise add the DMF solution of 4-amino-azobenzene (0.76g is dissolved in 3mL DMF), continue to maintain the temperature reaction for 2h, and then use 5 times the volume of diethyl ether was settled, filtered, and vacuum-dried to const...
Embodiment 2
[0059] Preparation of PU-CD-AB:
[0060] Dissolve 50g of polyethylene glycol, 6.75g of 2,3-dihydroxypropionaldehyde and 0.22g of dibutyltin dilaurate in 95mL of DMF, stir evenly, add 14.6g of hexamethylene diisocyanate, and mechanically stir , react at 80°C for 3.2h until the infrared characteristic absorption peak of -NCO disappears. 5 times the volume of glacial ether was settled, and vacuum-dried to obtain polyurethane PU-AG-II containing aldehyde groups in the side chain (the weight-average molecular weight was 4.8×10 4 g / mol);
[0061] Dissolve 10g of PU-AG-II in 20mL of DMF, cool down to 17°C, and add monoamino-β-cyclodextrin (β-CD-NH 2 ) DMF solution (5.96g dissolved in 5mL DMF), maintain the temperature reaction for 2h, continue to dropwise add the DMF solution of 4-amino-azobenzene (1.04g dissolve in 3mL DMF), continue to maintain the temperature reaction for 1.5h, and then use 5 times the volume of diethyl ether was settled, filtered, and vacuum-dried to constant ...
Embodiment 3
[0065] Preparation of PU-CD-AB:
[0066] Dissolve 50g of polyethylene glycol, 9.0g of 2,3-dihydroxypropionaldehyde and 0.25g of dibutyltin dilaurate in 100mL of DMF, stir evenly, add 18.85g of hexamethylene diisocyanate, and mechanically stir , React at 80°C for 3.5h until the characteristic infrared absorption peak of -NCO disappears. 5 times the volume of glacial ether was settled, and vacuum-dried to obtain polyurethane PU-AG-III containing aldehyde groups in the side chain (the weight-average molecular weight was 6.4×10 4 g / mol);
[0067] Dissolve 10g of PU-AG-II in 15mL of DMF, cool down to 18°C, and add monoamino-β-cyclodextrin (β-CD-NH 2 ) DMF solution (7.28g dissolved in 7mL DMF), maintain the temperature reaction for 2h, continue to dropwise add the DMF solution of 4-amino-azobenzene (1.26g dissolve in 3mL DMF), continue to maintain the temperature reaction for 1.5h, and then use 5 times the volume of diethyl ether was settled, filtered, and vacuum-dried to constan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com