Composite solid electrolyte material and preparation method and application thereof
A technology of solid electrolyte and electrolyte material, which is applied in the field of -zBN composite solid electrolyte material and its preparation, can solve the problems of low room temperature conductivity, unsatisfactory stability and electrode compatibility, low ionic conductivity, etc., and achieve improved ionic conductivity. The effect of conductivity, good compatibility and electrochemical stability, and strong lithium dendrite suppression ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] (1) In the isolation of air (H 2 O2 <1ppm), put 0.5g of hexagonal boron nitride into a stainless steel spherical tank equipped with stainless steel grinding balls; use a planetary wheel ball mill mechanical ball milling method, under the protection of high-purity (99.9999%) inert gas (argon) Next, hexagonal boron nitride with amorphous structure was obtained. The volume of the ball mill jar is 100 ml, the weight ratio of the balls to the sample is 40:1, the milling time is 10 hours, and the revolution speed is set at 500 rpm.
[0048] (2) Calculate the mass of hexagonal boron nitride and lithium borohydride with amorphous structure according to the molar ratio of 1:1, 1:2 and 1:3 respectively, weigh them and put them into a spherical tank with stainless steel grinding balls again The mixture of hexagonal boron nitride and lithium borohydride containing amorphous structure is obtained by adopting the mechanical ball milling method of planetary wheel ball mill under the ...
Embodiment 2
[0050] (1) In the isolation of air (H 2 O2 <1ppm), put 0.5g of hexagonal boron nitride into a stainless steel spherical tank equipped with stainless steel grinding balls; use a planetary wheel ball mill mechanical ball milling method, under the protection of high-purity (99.9999%) inert gas (argon) Next, hexagonal boron nitride with amorphous structure was obtained. The volume of the ball mill jar is 100 ml, the weight ratio of the ball to the sample is 45:1, the milling time is 20 hours, and the revolution speed is set to 450 rpm
[0051] (2) In the isolation of air (H 2 O2 4 Put it into a stainless steel spherical tank equipped with stainless steel grinding balls; adopt a planetary wheel ball mill mechanical ball milling method, under the protection of high-purity (99.9999%) inert gas (argon), to obtain LiBH 4 Mixture with LiI. The volume of the ball mill jar is 100 ml, the weight ratio of the balls to the sample is 40:1, the milling time is 4 hours, and the revolution sp...
Embodiment 3
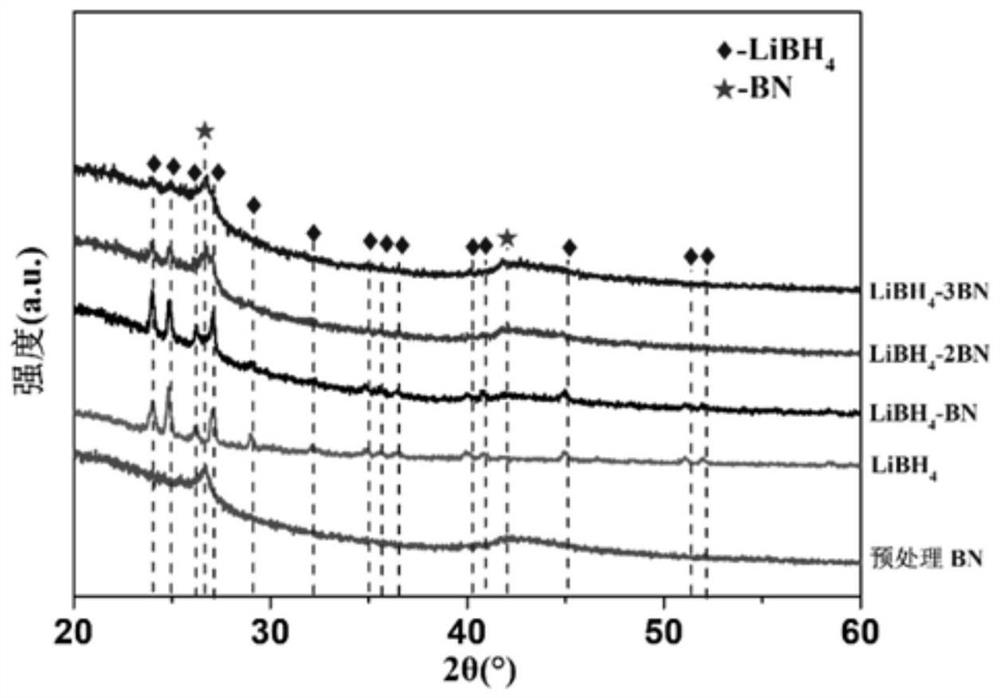
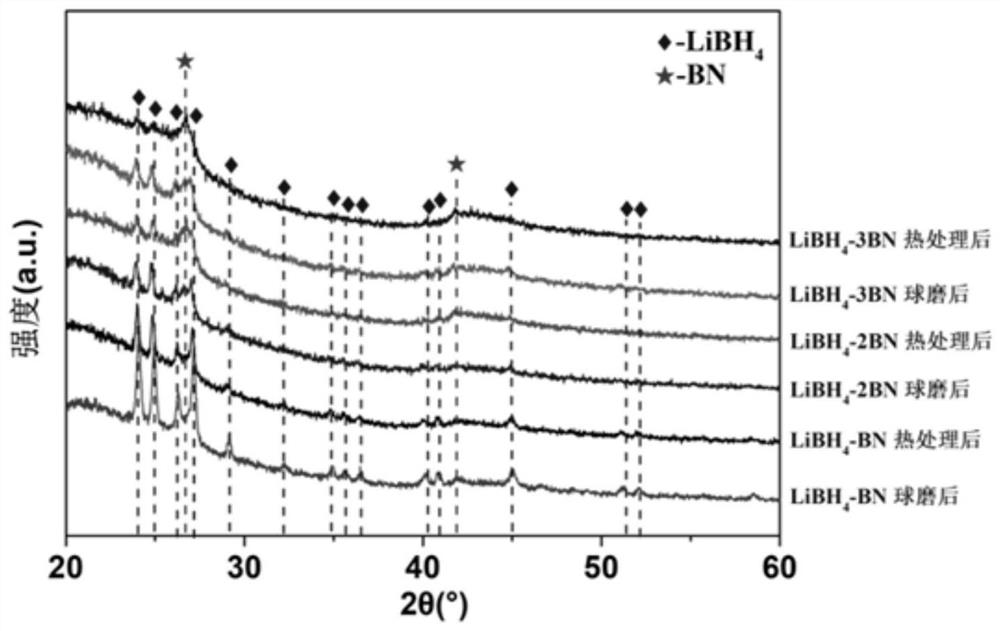
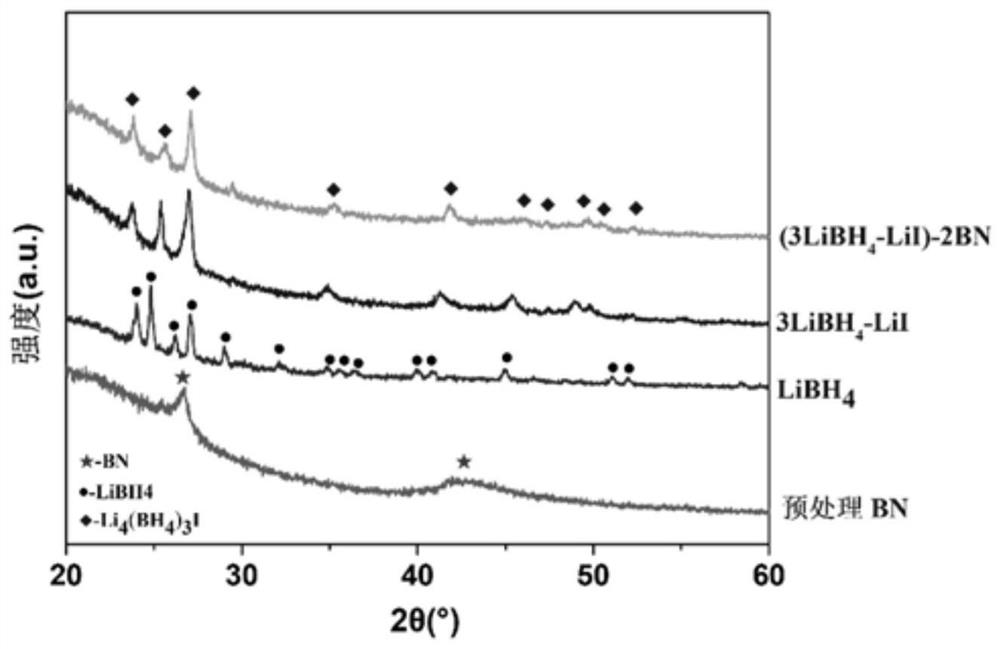
[0054] Get the LiBH prepared in Example 1 4 -zBN, z=1,2,3 and embodiment 2 prepare (3LiBH 4 -LiI)-zBN, z=1, 2, 3, 4, 5 for X-ray diffraction (XRD) experiments, the sample cell is covered by a specific polymer film, and it is sealed with a glass slide with vacuum grease to prevent air The effect of water and oxygen on the sample. The target material of the X-ray source used is a Cu target, the tube voltage is 40kV, and the tube current is 40mA.
[0055] see figure 1 and figure 2 , LiBH after ball milling and heat treatment 4 -zBN complex (z=1,2,3), the pre-milled h-BN phase peak position did not change, and the peak intensity was weakened compared with that before ball milling, indicating that there was some amorphization; LiBH 4 The same peak position does not change, and the peak intensity weakens, indicating that there is also some amorphization. The product has no mesophase formation, indicating that LiBH 4 There is no interaction with h-BN and no self-decomposition...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com