Application of umbilical cord stem cell freeze-dried powder in preparation of medicines and cosmetics
A technology for freeze-dried powder and cosmetics, which is applied in the directions of cosmetics, cosmetic preparations, drug combinations, etc., can solve the problems of complex components of freeze-dried powder, and achieve good effects and application values.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
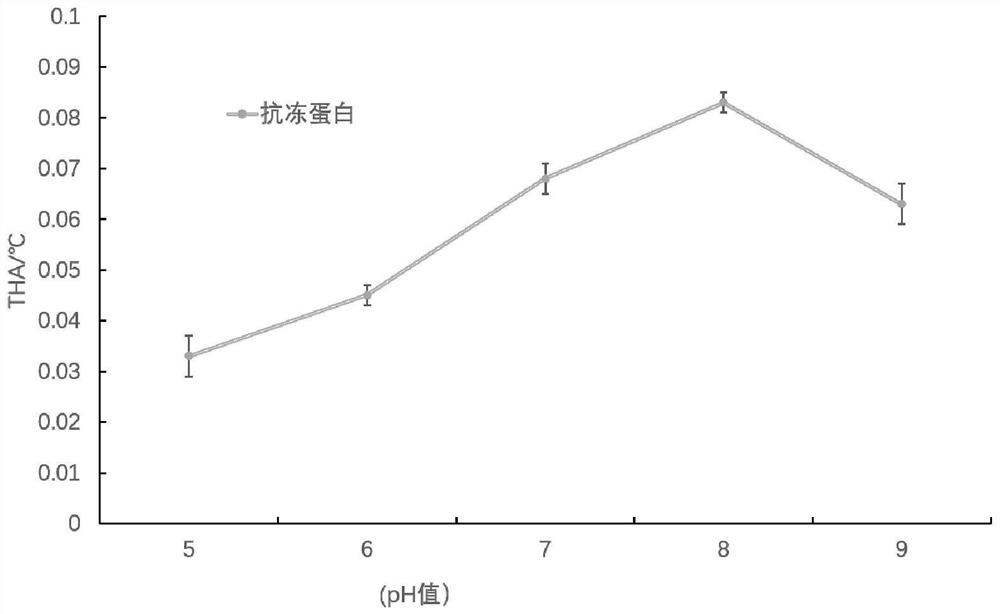
[0026] The screening of embodiment 1 antifreeze protein
[0027]Firstly, the Marigold officinalis was freeze-dried, ground and crushed, and stirred in 3 times the volume of 10 mmol / L PBS (pH 8.0) for 3 hours. The suspension is centrifuged at 3000r / min for 30min; the supernatant is precipitated with 50% to 100% saturated ammonium sulfate, the salting-out solution is centrifuged at 3000r / min for 30min, the precipitate is dialyzed against pure water overnight, and freeze-dried, called JZH antifreeze crude protein. JZH antifreeze crude protein 100mg was dissolved in 5mL 10mmol / L PBS (pH8.0), and separated by cation exchange column (2.6cm×50cm). The sample volume was 4 mL, and eluted with 10 mmol / L PBS (pH8.0) for 1 h, and then eluted with 0-1.5 mol / L NaCl (containing 10 mmol / L PBS, pH 8.0) for 5 h at a flow rate of 1.0 mL / min. The detection wavelength is 220nm. The active components showing THA were collected, dialyzed against pure water, and freeze-dried. 150mg of lyophilized...
Embodiment 2
[0031] Embodiment 2 Identification of antifreeze characteristics
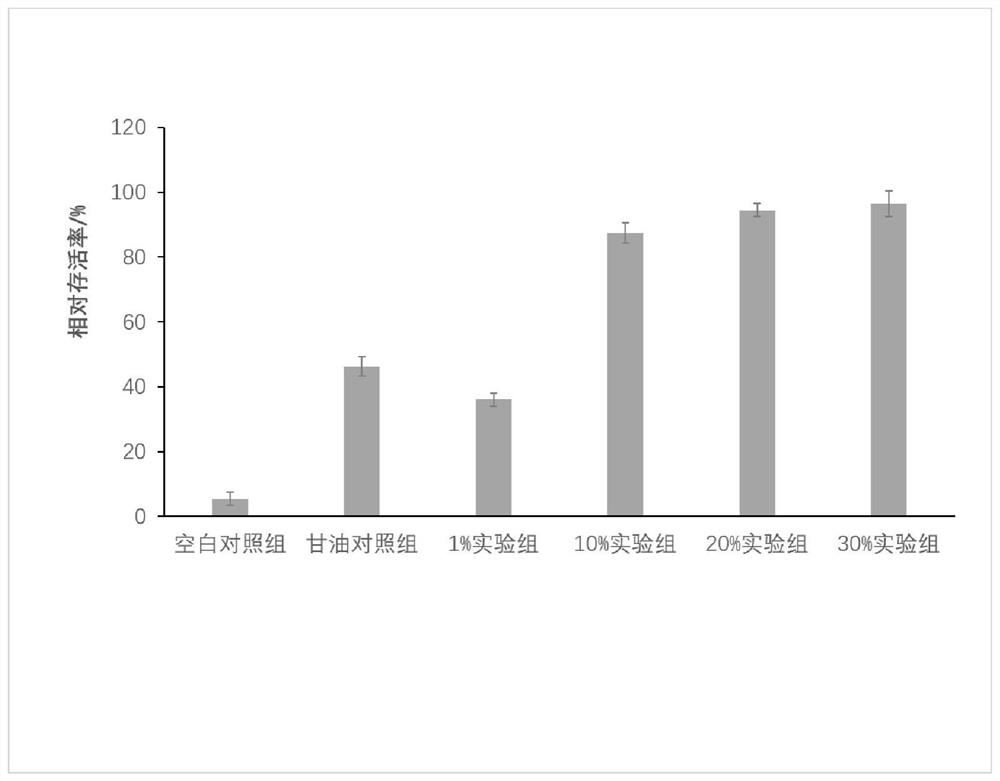
[0032] For the detection of the activity of Escherichia coli added with antifreeze protein, take freshly activated Escherichia coli liquid, cultivate it until the absorbance OD600 is about 1.0, and dilute it with sterile water for 10 4 times, dispensed into sterilized EP tubes, added the purified antifreeze protein of SEQ ID NO: 1 and mixed with the bacterial solution to a final concentration of 1%, 10%, 20%, and 30% W / v, and at the same time The blank bacterial solution and the bacterial solution added with 20% glycerol were used as the control, and all the experimental groups were placed at -10°C, frozen for 120 hours, 50uL of the bacterial solution was drawn and spread on the LB plate (3 replicates for each group), and incubated upside down at 37°C for 24 hours Afterwards, count the colonies. The result is as figure 2 shown.
[0033] From figure 2 It can be seen from the results that the bacterial surv...
Embodiment 3
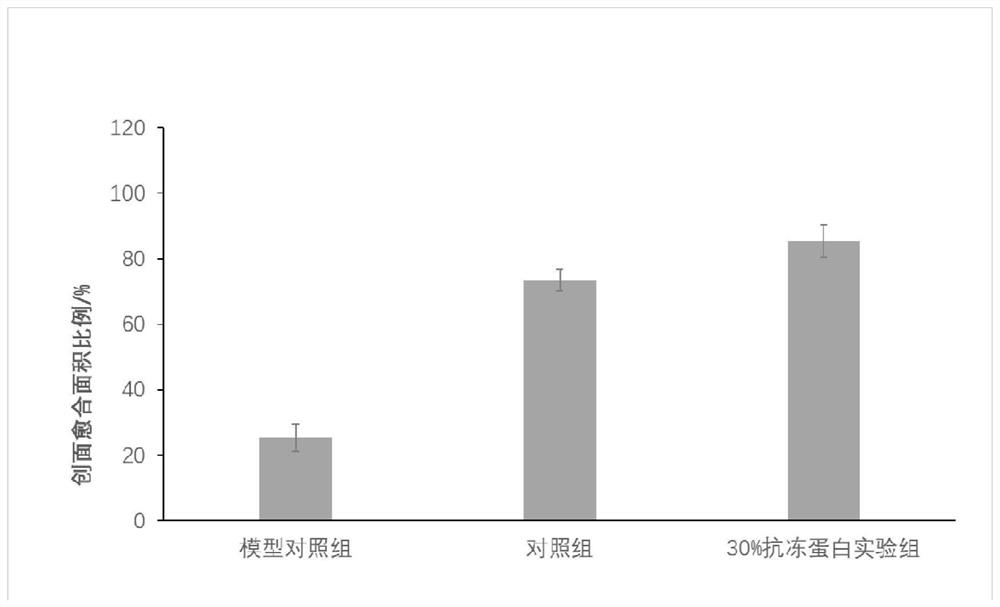
[0034] Embodiment 3 Preparation of umbilical cord stem cells
[0035] Under sterile conditions, 50ml of umbilical cord blood was taken, and the concentration of heparin was 20U / ml for anticoagulation. Dilute and mix with Hanks balanced salt solution, add Ficoll-Paque medium, the column height ratio of diluted blood to Ficoll-Paque medium is 2:1, centrifuge at 2000r / min for 20min, take the mononuclear cells in the interface layer, Add Hank's balanced salt solution to centrifuge, wash twice, add to the culture medium, and adjust the cell concentration to about 106 / ml. The medium was Mesencult TM medium (Stem Cell Company), and its pH value was adjusted to be acidic, and 1% Pen strep (Gibco, Grand Island, NY) was added, and the cells were divided into 25T culture flasks and placed at 37°C. After culturing in an incubator with saturated humidity and a volume fraction of 5% CO2 for 72 hours, replace the culture medium, discard the unattached cells, and change the medium once every...
PUM
| Property | Measurement | Unit |
|---|---|---|
| absorbance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com