Diamine monomer containing tetraphenylethylene-bis-arylamine structure, preparation method and application of diamine monomer in super-stable electrochromic material
A technology of tetraphenylethylene and diamine monomers, which is applied in the direction of color-changing fluorescent materials, preparation of organic compounds, preparation of nitro compounds, etc., can solve the influence of material stability, high electrochromic oxidation potential, and limit black electrochromic The application of color-changing materials and other problems can reduce the oxidation potential and improve the stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
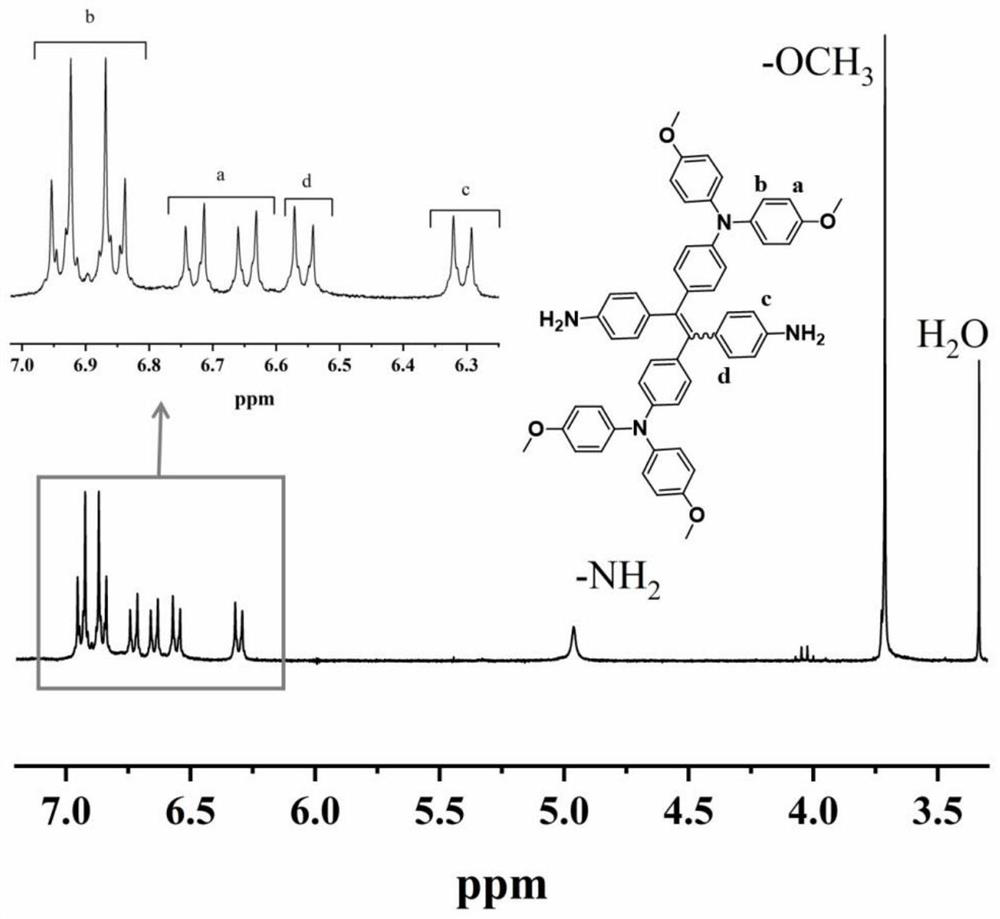
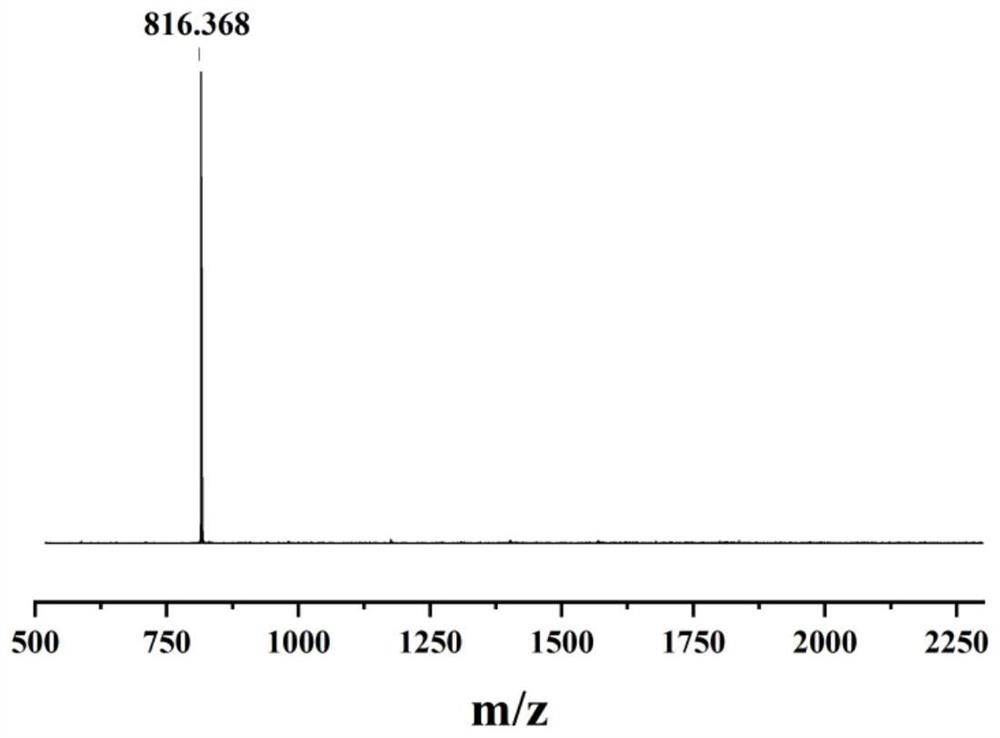
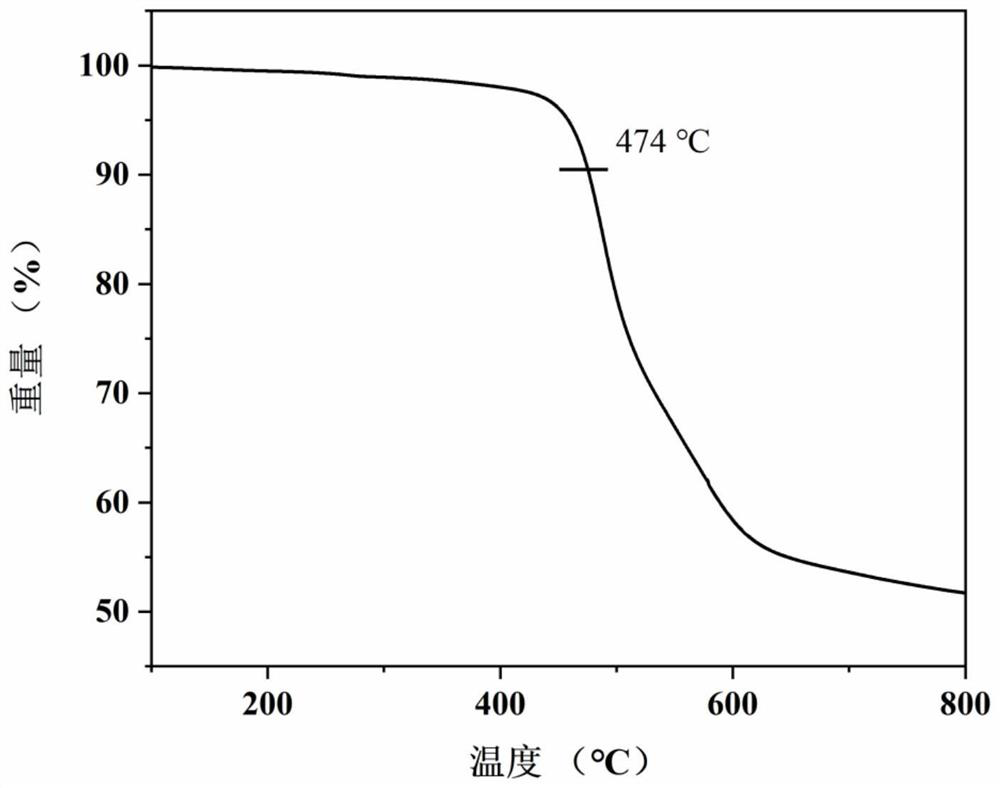
[0035] Example 1: N-{4-[1,2-bis(4-aminophenyl)-2-{4-[bis(4-methoxyphenyl)amino]phenyl}vinyl]phenyl} Preparation of -4-methoxy-N-(4-methoxyphenyl)aniline
[0036] The first step reaction: add 24g of 4-bromobenzophenone, 15.7g of zinc powder to a 1000mL three-necked flask equipped with mechanical stirring, cool to -78°C after 500mL of tetrahydrofuran, stir and slowly drip under nitrogen protection Add 23g of titanium tetrachloride, return to room temperature and then heat to 85°C for 24h reaction. After the mixture was cooled to room temperature again, the reaction was quenched with 500 mL, 10 wt% aqueous potassium carbonate solution, the organic layer was collected after filtration, the aqueous layer of the filtrate was extracted three times with ethyl acetate, the organic phases were combined and dried with magnesium sulfate overnight, and the ethyl acetate was evaporated. After mixing with tetrahydrofuran, a solid crude product was obtained. The crude product was recrystalli...
Embodiment 2
[0041] Example 2: N-{4-[1,2-Bis(4-aminophenyl)-2-{4-[bis(4-methoxyphenyl)amino]phenyl}vinyl]phenyl} -4-Methoxy-N-(4-methoxyphenyl)aniline to prepare pyromellitic dianhydride type polyimide.
[0042] To a three-necked flask equipped with a magnetron, a nitrogen inlet and outlet, and a thermometer, add 0.290 g of N-{4-[1,2-bis(4-aminophenyl)-2-{4- [bis(4-methoxyphenyl)amino]phenyl}vinyl]phenyl}-4-methoxy-N-(4-methoxyphenyl)aniline and 0.078 g of pyromellitic acid bis anhydride, added 4 mL of N,N-dimethylacetamide (solid content 27%), reacted at room temperature for 24 h to obtain a viscous polyamic acid, then added 1.4 mL of acetic anhydride and 0.7 mL of pyridine, heated to 110 °C and reacted for 3 h , after the reaction is completed, cooled to room temperature, discharged into ethanol to obtain a white fibrous product, washed with ethanol under reflux for 30 min, washed with water under reflux, washed with ethanol under reflux for 30 min, and dried in a vacuum oven at 90° C. ...
Embodiment 3
[0044] Example 3: N-{4-[1,2-Bis(4-aminophenyl)-2-{4-[bis(4-methoxyphenyl)amino]phenyl}vinyl]phenyl} Preparation of polyimide by polymerization of -4-methoxy-N-(4-methoxyphenyl)-p-3,3',4,4'-benzophenone tetraacid dianhydride
[0045] To a three-necked flask equipped with a magnetron, nitrogen inlet and outlet, and a thermometer, add 0.29 g of N-{4-[1,2-bis(4-aminophenyl)-2-{4- [bis(4-methoxyphenyl)amino]phenyl}vinyl]phenyl}-4-methoxy-N-(4-methoxyphenyl)aniline and 0.112 g of 3,3', 4,4'-benzophenone tetraacid dianhydride, added 4mL of N,N-dimethylacetamide, reacted at room temperature for 24h to obtain a viscous polyamic acid, then put in 1.4mL of acetic anhydride and 0.7mL of pyridine, heated up The reaction was carried out at 110 °C for 3 h. After the reaction was completed, it was cooled to room temperature and discharged into ethanol to obtain a yellow-white fibrous product. The ethanol was refluxed for 30 min, washed once with water, washed with ethanol under reflux for 30...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com