Novel podophyllotoxin spliced anti-tumor active molecular compound as well as preparation method and application thereof
An anti-tumor activity, molecular compound technology, applied in the field of chemistry, can solve the problems of multidrug resistance gastrointestinal function, narrow anti-tumor spectrum, disorder and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
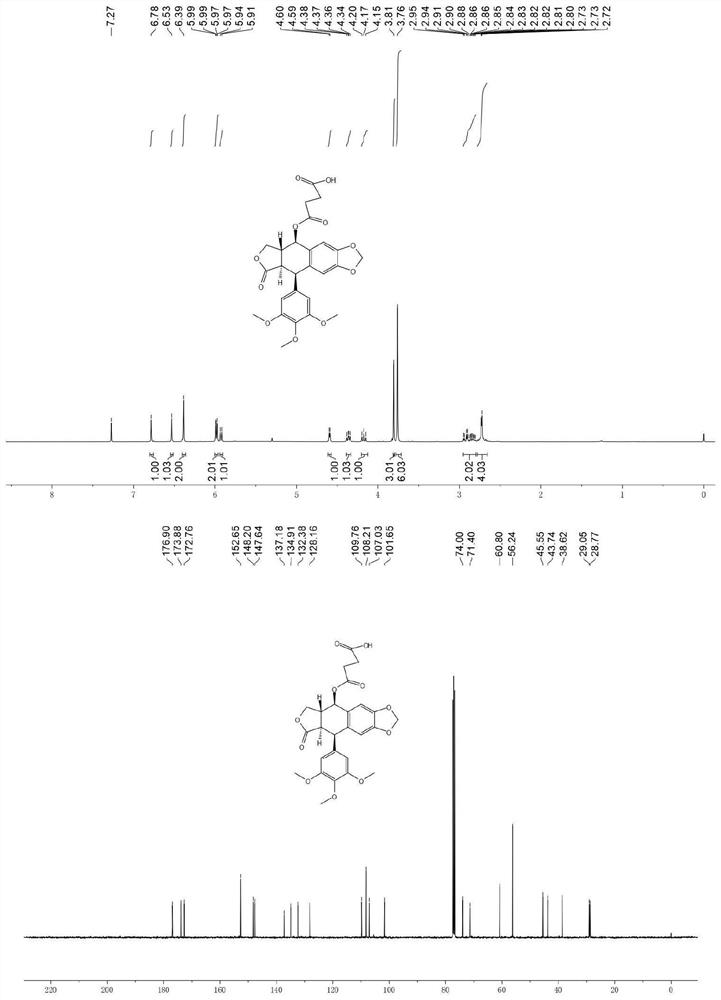
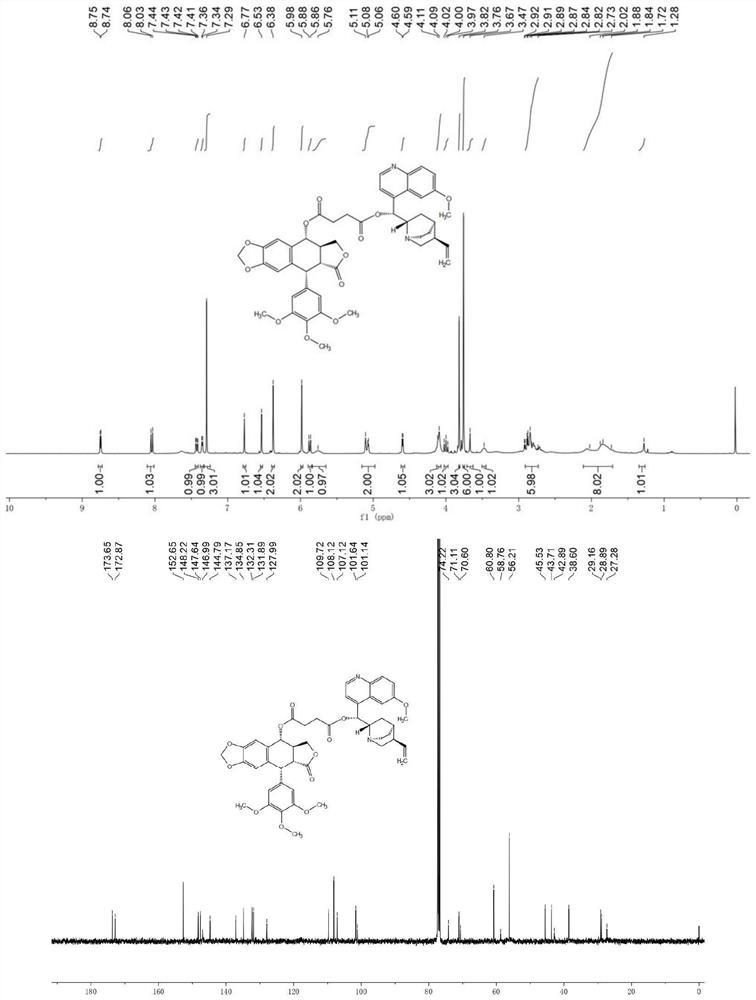
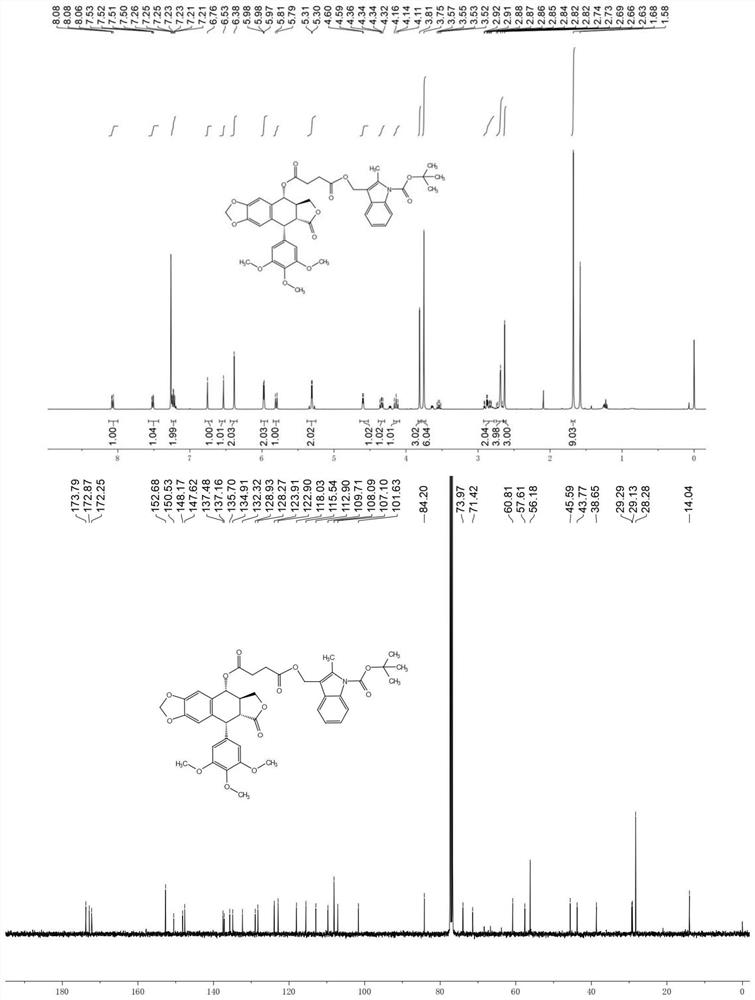
[0028] Example of the present invention: 414.41mg podophyllotoxin (1mmol), 200.1mg succinic anhydride (2eq, 2.0mmol), 101.2mg acid binding agent triethylamine, 122.1mg catalyst 4-dimethylamino were sequentially added to the reaction tube Pyridine and 5 ml of dichloromethane solution were reacted at room temperature for 2 hours, TLC detected that the reaction was basically complete, the solvent was spin-dried, and the sample was loaded and subjected to silica gel column chromatography (eluent: V (dichloromethane): V (methanol) = 100:3 ) was purified to obtain the intermediate compound 1a. White solid, melting point: 75.5–76.4°C; yield 99.2%; NMR and high-resolution mass spectrometry test results are as follows: 1 H NMR (CDCl 3 ,400MHz)δ:6.78(s,1H),6.53(s,1H),6.39(s,2H),5.98(dd,J=6.2,1.3Hz,2H),5.93(d,J=9.1Hz,1H ),4.60(d,J=4.4Hz,1H),4.36(dd,J=9.3,6.9Hz,1H),4.17(t,J=9.8Hz,1H),3.81(s,3H),3.76(s ,6H),2.97–2.78(m,2H),2.75–2.68(m,2H). 13 C NMR (CDCl 3 ,100MHz)δ:176.90,173.88,172....
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com