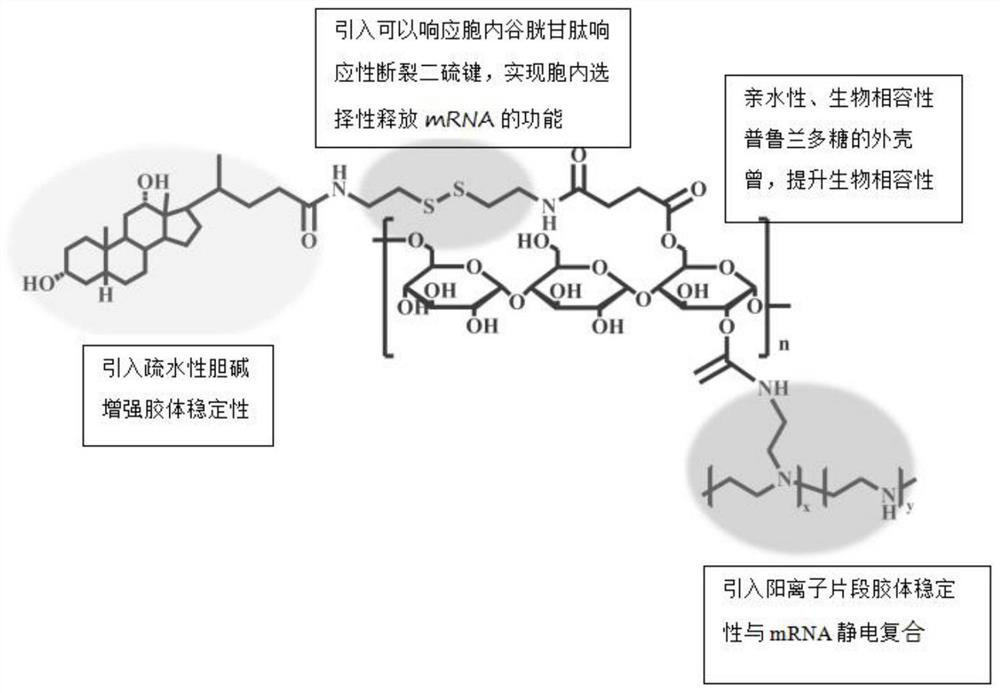
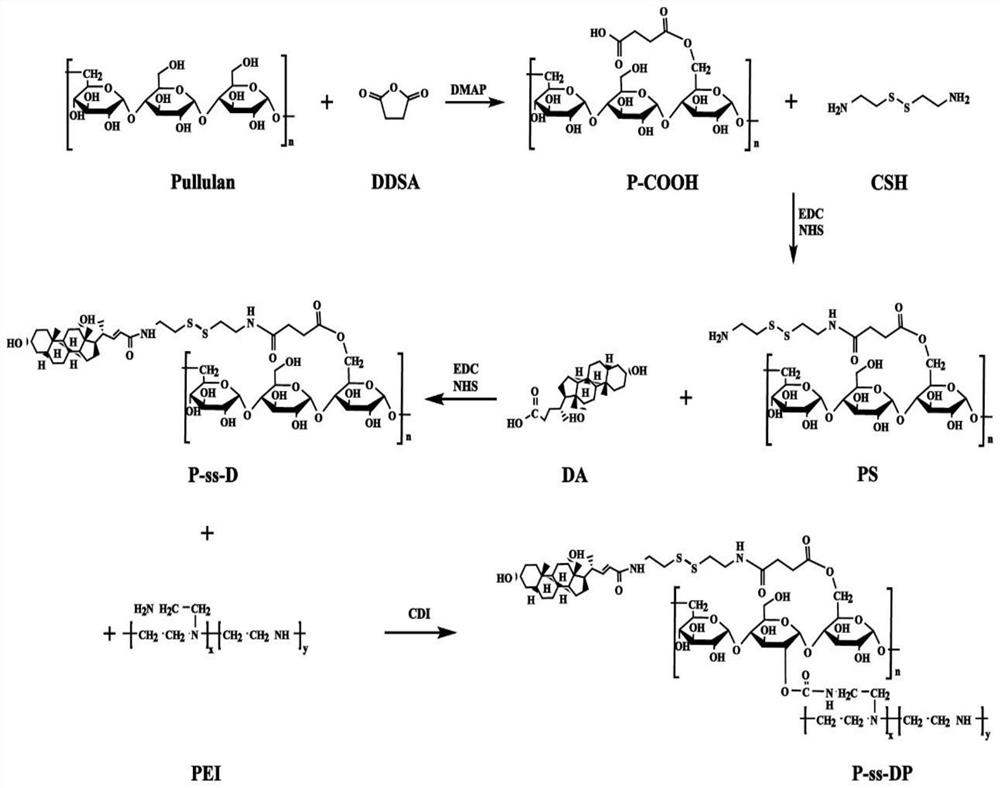
MRNA transfection material, mRNA transfection system and application
A transfection system and reaction technology, applied in the biological field, can solve the problem that the system cannot be too stable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Synthesis of mRNA transfection material P-ss-DP of the present invention
[0046] (1) Synthesis of P-COOH: succinylated pullulan (P-COOH) was synthesized by esterification of succinic anhydride with pullulan (P). The specific synthesis steps are:
[0047] Dissolve 1.62 g of pullulan in DMSO, add 0.2 g of DMAP and 0.8 g of succinic anhydride, and continue stirring for 24 h on a magnetic stirrer at 45°C. After the reaction, the reaction solution was added to 500 mL of anhydrous ethanol and vigorously stirred, and a white precipitate appeared and centrifuged to remove the anhydrous ethanol. After collecting the reaction product, it was dispersed and dissolved in 10 mL of deionized water. After 3 days of dialysis in deionized water using a dialysis bag (MWCO, 10 kDa), the dialyzed solution was freeze-dried to obtain a white flocculent solid P-COOH.
[0048] (2) Synthesis of PS: Dissolve 1.42 g of P-COOH in 100 mL of PBS (0.2 M, pH 7.8), add 381.3 mg of NHS and 6...
Embodiment 2
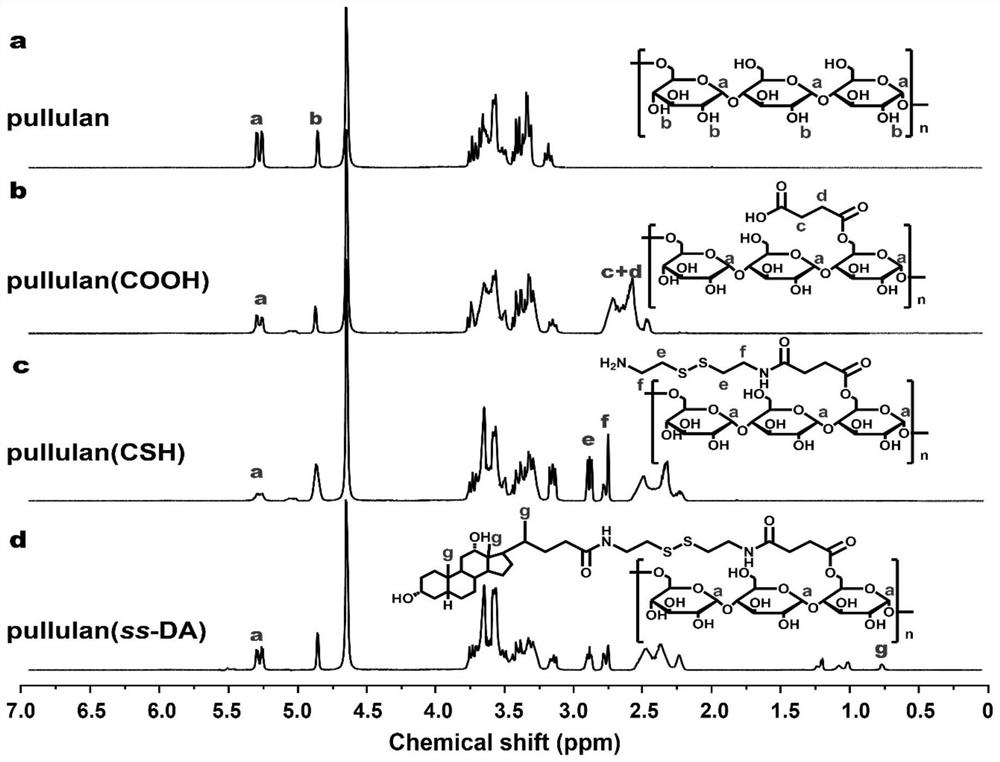
[0051] Example 2 Structural analysis of the mRNA transfection material of the present invention
[0052] The structures of pullulan and its derivatives were analyzed by 1HNMR. like image 3 As shown, relative to the hydrogen spectrum of Pullulan, the carboxylated pullulan P-COOH appears between 2.4-2.7 ppm corresponding to the methylene group (C-CH) on succinic anhydride. 2 -CH 2 -C) of the new proton absorption peak. The degree of substitution of succinic anhydride on the sugar chain of pullulan (average number of substituents per 100 sugar units) was about 29 detected by acid-base titration. Cystamine methylene (NH-CH 2 -CH 2 -S) The chemical shift of the proton peak is between 2.8 and 2.9 ppm. The degree of substitution of cystamine is estimated to be about 18.5 by the integrated area of the proton peak, and the degree of substitution of cystamine is estimated to be about 17.6 by elemental analysis. In the P-ss-D hydrogen spectrum, new proton peaks appear at 0.5-1.4...
Embodiment 3
[0053] Example 3 Preparation of nucleic acid delivery system (P-ss-DP / mRNA nanoparticle complex)
[0054] Weigh an appropriate amount of the P-ss-DP polymer powder of Example 1 and dissolve it in Tris-HCl buffer (10 mM, pH 7.4). Phosphorus ratio, the ratio of the amount of the positive charge provided by the amino group in the carrier molecule to the amount of the negatively charged substance provided by the phosphate group in the nucleic acid molecule) was 1, 5, 10, 20, 30, added to the mRNA solution, and vortexed. Homogenize, and adjust the mRNA concentration in the polymer & mRNA complex formed by the final self-assembly to 33.3 ng / μL. The mixed P-ss-DP / mRNA nanoparticles were incubated overnight at 4°C before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com