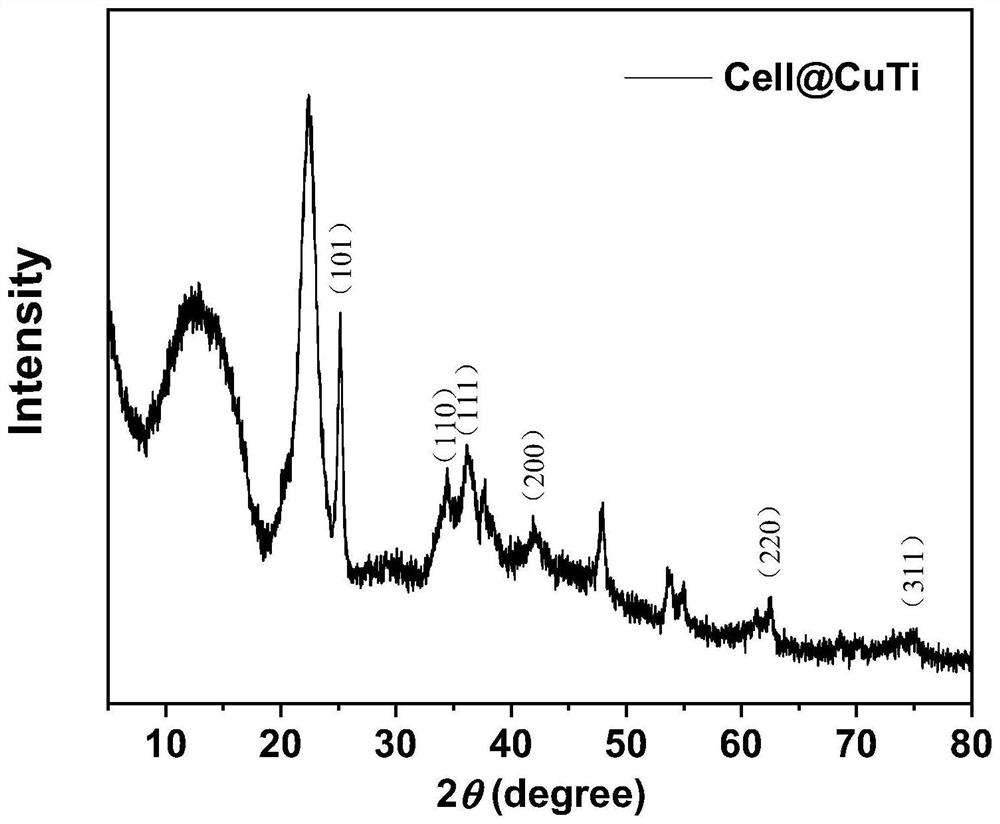
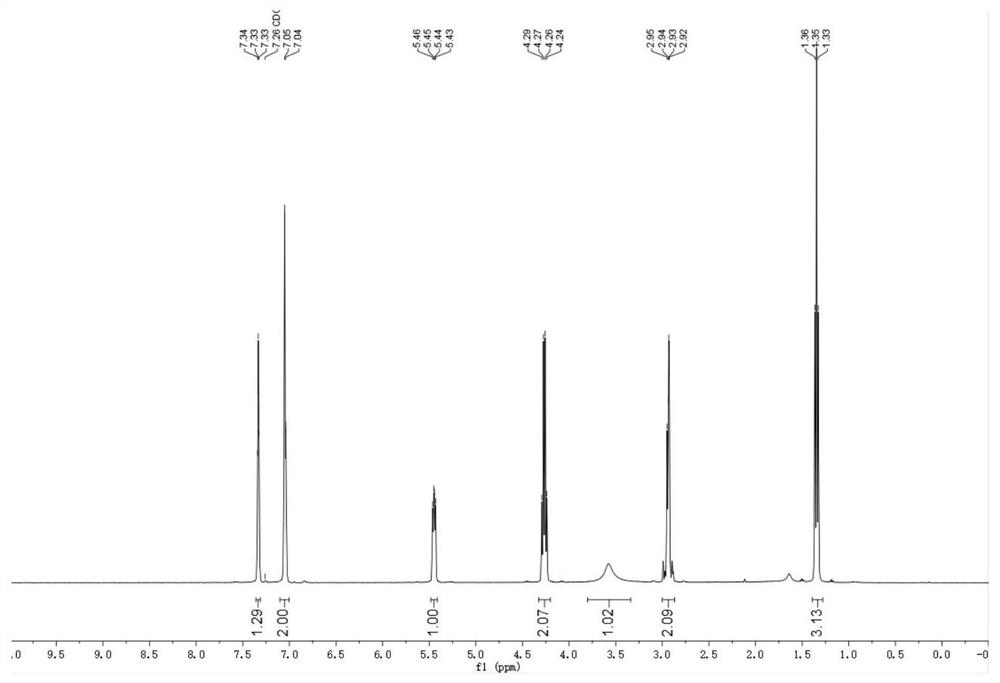
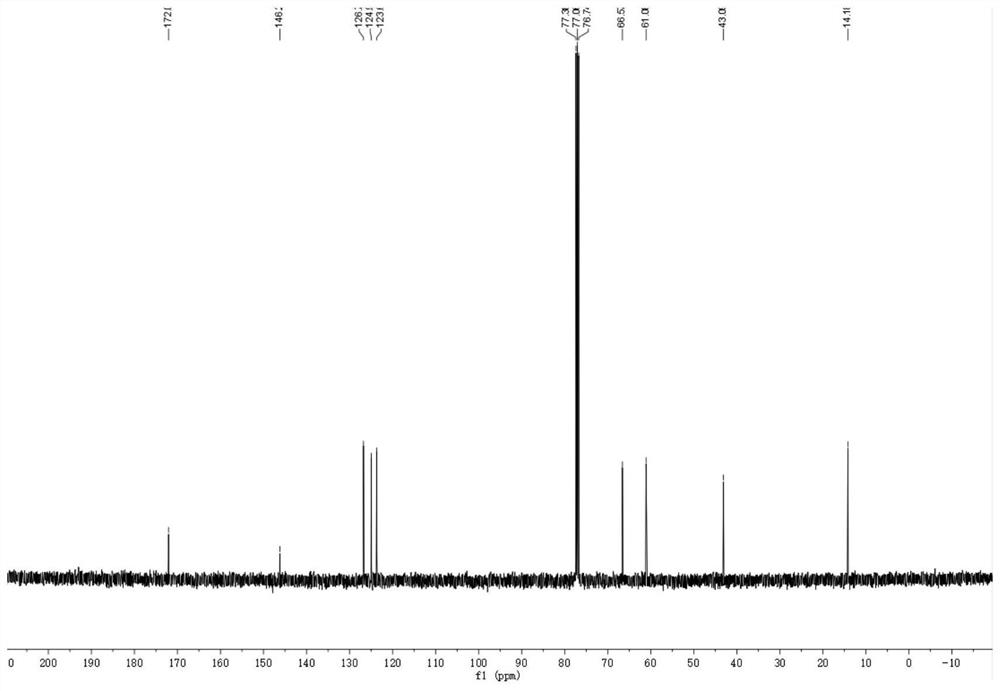
Cellulose-loaded heterojunction catalytic material and method for preparing chiral boride from cellulose-loaded heterojunction catalytic material
A catalytic material and cellulose technology, applied in the direction of catalyst activation/preparation, organic chemical methods, chemical instruments and methods, etc., can solve the problems of unrecyclable catalysts, high cost, and impossibility of industrialization, etc., and achieve high conversion rate and antipodal Selectivity, short reaction time, pollution reduction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The present embodiment provides a preparation method of a cellulose-supported metal oxide catalytic material, comprising the following steps:
[0045] a.TiO 2 Preparation of:
[0046] Configuration solution 1: Add 20ml of tetrabutyl titanate and 70ml of absolute ethanol into the beaker, stir for 20min, and form a yellow clear solution;
[0047] Configure solution 2: add 4 mL of glacial acetic acid, 20 mL of distilled water and 70 mL of absolute ethanol into a beaker, stir for 20 min, and dropwise add 1 mol / L HCl to control the pH between 3 and 4 to obtain solution 2;
[0048] Add solution 1 to solution 2, stir in a water bath at 50 °C for 1 h to obtain a white gel, dry the white gel in an oven at 80 °C for 24 h to obtain yellow crystals, grind to obtain yellow powder, and calcine the yellow powder at 600 °C 2h, get TiO 2 powder;
[0049] b.Cu 2 O / TiO 2 preparation
[0050] Take 0.0802g NaOH and add it to 20mL distilled water, stir and dissolve to obtain NaOH solu...
Embodiment 2
[0056] This embodiment provides the application of the catalytic material Cell@CuTi in the boron addition reaction of α,β-unsaturated ester I and pinacol biboronate reagent, including the following steps:
[0057] 1) Add α,β-unsaturated ester I, pinacol biboronate, catalytic material Cell@CuTi (prepared in Example 1) and ligand (R,S)-josiphos into 0.2 ml of toluene to prepare Dissolve, then add 1.8ml water and stir at room temperature for 3h, the asymmetric boronation reaction of α,β-unsaturated ester occurs, wherein α,β-unsaturated ester I 0.20mmol, biboronic acid pinacol ester 0.24mmol, catalytic The material Cell@CuTi (10.0mg) contains 0.002mmol of copper and 0.002mmol of ligand (R,S)-josiphos. In this example, α,β-unsaturated ester I is 2-ene-3-thiophene propionate ethyl ester, Wherein R is a thienyl group, and the reaction formula is as follows:
[0058]
[0059] 2) After the reaction, the reaction solution was filtered, and the obtained precipitate was repeatedly was...
Embodiment 3
[0081] This embodiment provides the application of the catalytic material Cell@CuTi in the boron addition reaction of α,β-unsaturated ester I and pinacol biboronate reagent, including the following steps:
[0082] 1) Add α,β-unsaturated ester I, pinacol biboronate, catalytic material Cell@CuTi (prepared in Example 1) and ligand (R,S)-josiphos into 0.2 ml of toluene to prepare Dissolve, then add 1.8ml water and stir at room temperature for 3h, the asymmetric boronation reaction of α,β-unsaturated ester occurs, wherein α,β-unsaturated ester I 0.20mmol, biboronic acid pinacol ester 0.24mmol, catalytic The material Cell@CuTi (10.0mg) contains 0.002mmol of copper and 0.002mmol of ligand (R,S)-josiphos. In this example, α,β-unsaturated ester I is 2-ene-3-p-chlorophenylpropionic acid Ethyl ester, wherein R is p-chlorophenyl, the reaction formula is as follows:
[0083]
[0084] 2) After the reaction is completed, the reaction solution is filtered, the obtained precipitate is repe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com