Method for detecting residual impurities in entacapone
A technology of residual impurities and entacapone, which is applied in the field of detection of residual impurities in entacapone, can solve the problems of effective detection of N,N-diethylcyanoacetamide, etc., and achieves high sensitivity, high precision, Easy to use effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Exclusive test:
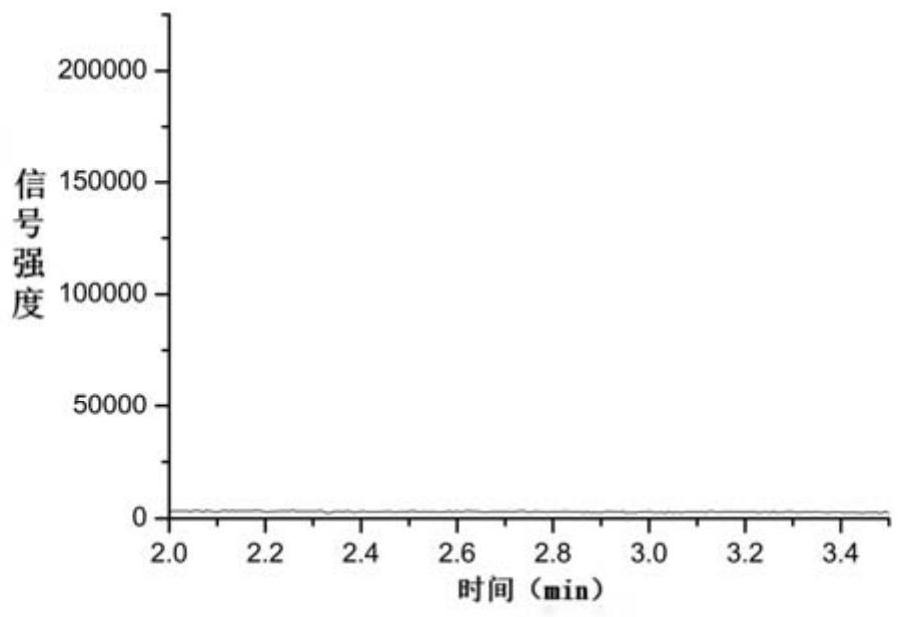
[0058] Blank solution: pure water.
[0059] Reference substance stock solution: take an appropriate amount of N,N-diethylcyanoacetamide, accurately weigh it, and prepare a N,N-diethylcyanoacetamide solution containing about 2.00 μg per 1 mL of water, as the reference substance stock solution .
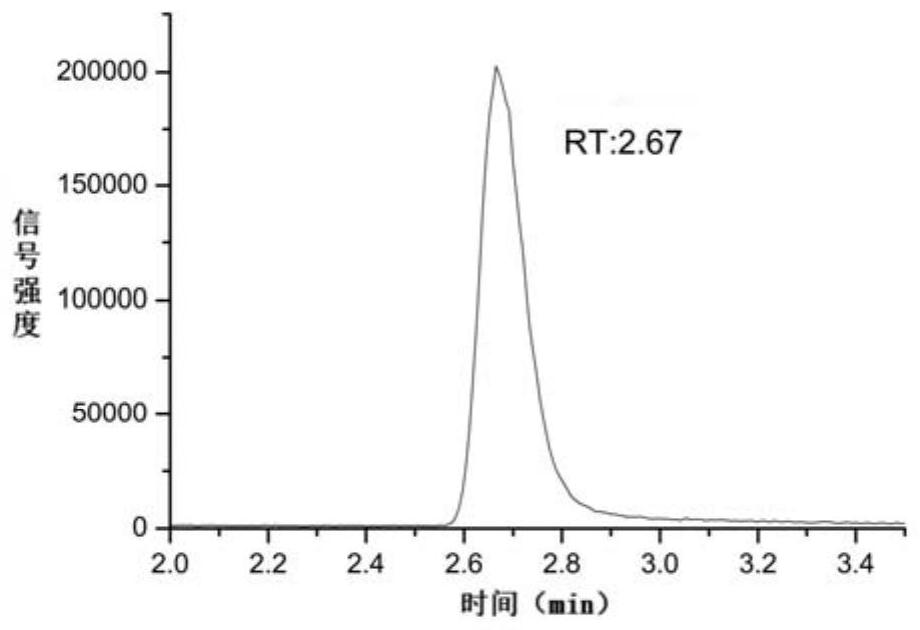
[0060] Reference substance solution: Take an appropriate amount of the reference substance stock solution, accurately weigh it, and dilute it with water to about 100ng of N,N-diethylcyanoacetamide solution per 1mL, as the reference substance solution.
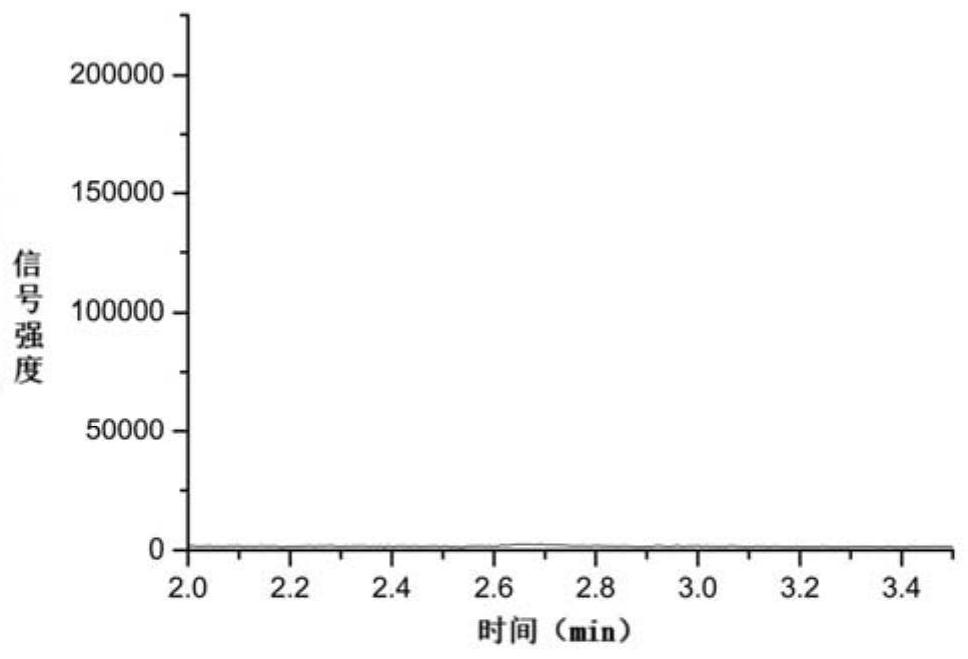
[0061] Test solution: take about 25.00mg of entacapone raw material, accurately weigh it, put it in a 250mL volumetric flask, add water to dissolve and dilute to the mark, as the test solution.
[0062] Test substance plus reference substance solution: take about 25.00mg of entacapone raw material, accurately weigh it, put it in a 250mL volumetric flask, add 12.5mL of reference substance stock solution, add wate...
Embodiment 2
[0066] Linear relationship:
[0067] Linear stock solution: take an appropriate amount of N,N-diethyl cyanoacetamide reference substance, accurately weigh it, and make a N,N-diethyl cyanoacetamide solution containing about 2.00 μg per 1 mL of water as a linear stock solution .
[0068] Linear solution: Precisely measure an appropriate amount of linear stock solution and dilute with water to a solution containing about 5.11ng, 20.44ng, 61.31ng, 102.19ng, 143.07ng and 204.38ng of N,N-diethylcyanoacetamide per 1mL, respectively. Shake well as a linear solution.
[0069] Take each linear solution, carry out HPLC-MS / MS detection, and record the chromatogram. Taking the injection concentration of N,N-diethylcyanoacetamide (ng / mL) as the abscissa and the peak area as the ordinate, draw a standard curve, such as Figure 5 ; And calculate the regression equation, the results are shown in Table 1.
[0070] Table 1 N,N-Diethylcyanoacetamide linearity test results
[0071]
[0072...
Embodiment 3
[0075] Limit of detection and limit of quantitation tests:
[0076] Reference substance stock solution: take an appropriate amount of N,N-diethylcyanoacetamide, accurately weigh it, and prepare a N,N-diethylcyanoacetamide solution containing about 2.00 μg per 1 mL of water, as the reference substance stock solution .
[0077] Reference substance solution: Take an appropriate amount of the reference substance stock solution, accurately weigh it, and dilute it with water to about 100ng of N,N-diethylcyanoacetamide solution per 1mL, as the reference substance solution.
[0078] Take the reference solution, quantitatively dilute it with water step by step, carry out HPLC-MS / MS detection, record the chromatogram, and obtain the detection limit according to the signal-to-noise ratio not less than 3:1, and according to the signal-to-noise ratio not less than 10:1, get The limit of quantification is shown in Table 2.
[0079] 6 copies of the limit of quantification solution were pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com