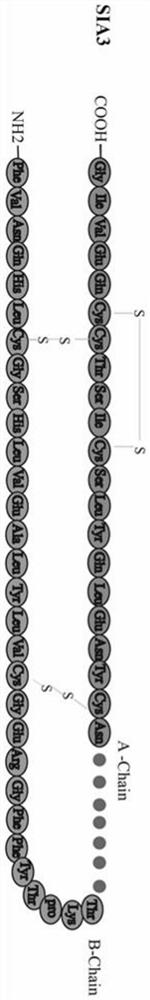
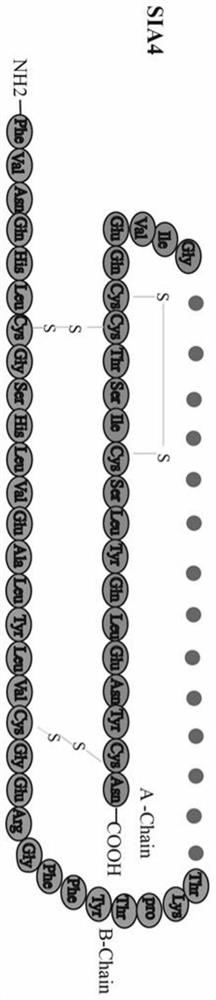
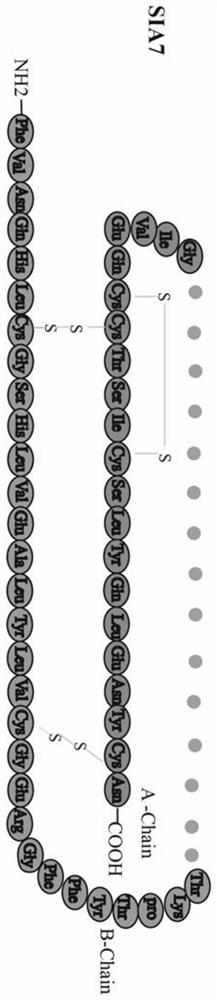
Human-derived single-chain insulin analogue and application thereof
A single-source technology for insulin analogues, applied in the field of human single-chain insulin analogues, can solve problems such as weak binding, complicated production process, and huge global burden of diabetes, and achieve the effect of efficient expression and wide use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] Example 1 ELISA detection of cells expressing SIA
[0090] (1) Return all reagents and components to room temperature before use;
[0091] (2) Set standard wells, 0-value wells, blank wells and sample wells. Add 50 μL of different concentrations of standard to each of the standard wells, add 50 μL of sample diluent to 0-value wells, leave blank wells, and add samples to be tested to sample wells 50 μL; except blank wells, standard wells, zero value wells and sample wells, add 100 μL of horseradish peroxidase (HRP)-labeled detection antibody;
[0092] (3) Cover the reaction plate with a sealing film and incubate at 37°C for 60 minutes;
[0093] (4) Remove the sealing film, discard the liquid, pat dry on absorbent paper, fill each well with washing solution, let stand for 20 s, shake off the washing solution, pat dry on absorbent paper, repeat 5 times; For the plate machine, please wash the plate according to the operation procedure of the plate washer. Adding a soaking...
Embodiment 2
[0097] Example 2 Detection of SIA and Antibody Binding Ability Expressed by Cells
[0098] (1) Cells HEK293T were evenly inoculated in a 10cm plate and cultured overnight;
[0099] (2) Transiently transfect HEK293T with plasmid pSC-SIA-his, collect the supernatant after 48 hours, and transfect with pSC plasmid as a control;
[0100] (3) Coat each well of ELISA plate overnight at 4°C with 0.1 μg of 1 μg / mL insulin antibody (anti-human insulin antibody, Youpin Bio YP100200), 100 μL per well; wash 5 times with PBST for 30 s each time;
[0101] (4) Add 100 μL of 1% BSA (prepared in PBS) to each well, block at 37°C for 2 hours; wash with PBST for 5 times, 30 s each time;
[0102] (5) Add 50 μL of standard or test sample and 50 μL of HRP-labeled human insulin at a concentration of 1.25 ng / ml, incubate at 37°C for 1 h (both diluted samples and antibodies are prepared with 1% BSA (PBST); PBST washes 5 times, 30 s each time ;
[0103] (6) Mix the chromogenic solutions A and B at a v...
Embodiment 3
[0106] Example 3 Detection of the binding ability of SIA expressed by cells to human insulin / mouse insulin receptors
[0107] (1) Cells HEK293T were evenly inoculated in a 10cm plate and cultured overnight;
[0108] (2) HEK293T was transiently transfected with plasmid pSC-SIA-his, and the supernatant was collected after 48 hours, concentrated and detected with Millipore's Amiconultra specification as a 3k ultrafiltration tube, and the concentration was adjusted for insulin antibody binding experiments. Plasmids were transfected as controls;
[0109] (3) Each well of the ELISA plate was coated with 1 μg / mL insulin receptor 0.1 μg (anti-human insulin antibody, Youpin Bio YP100200) overnight at 4°C, 100 μL per well; washed 5 times with PBST, 30 s each time;
[0110] (4) Add 100 μL of 1% BSA (prepared in PBS) to each well, block at 37°C for 2 hours; wash with PBST for 5 times, 30 s each time;
[0111] (5) Add 50 μL of SIA samples with different concentration gradients and 50 μL ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com