Application and preparation method of complex for catalyzing ring opening polymerization of lactide
A technology for catalyzing lactide and ring-opening polymerization, applied in the direction of magnesium organic compounds, chemical recovery, etc., can solve the problems of catalyst toxicity, product impurity, and high reaction conditions of chain extension method, and achieve narrow molecular weight distribution, simple preparation method, The effect of high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
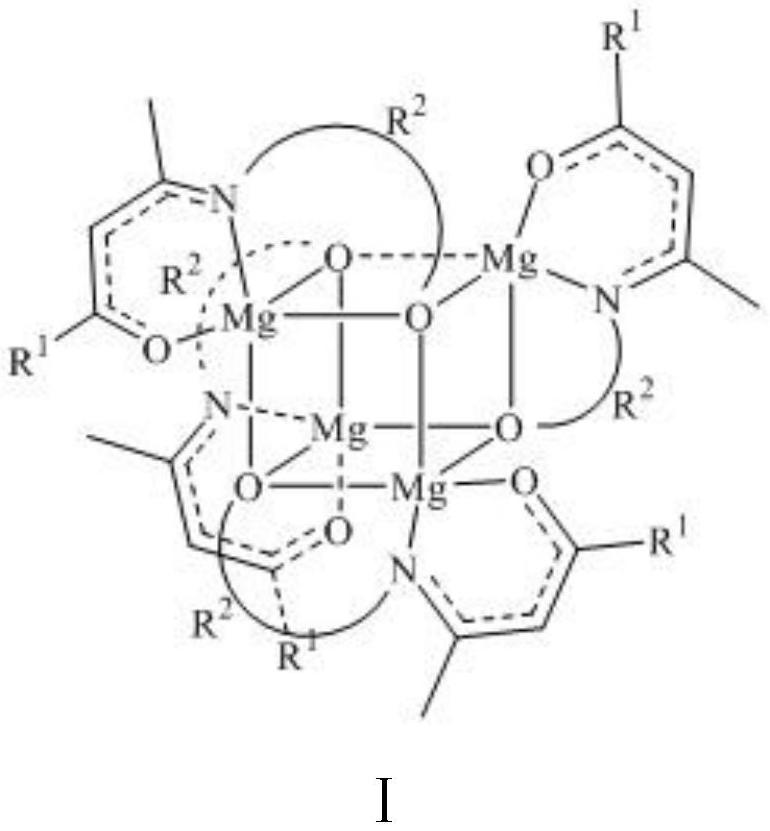
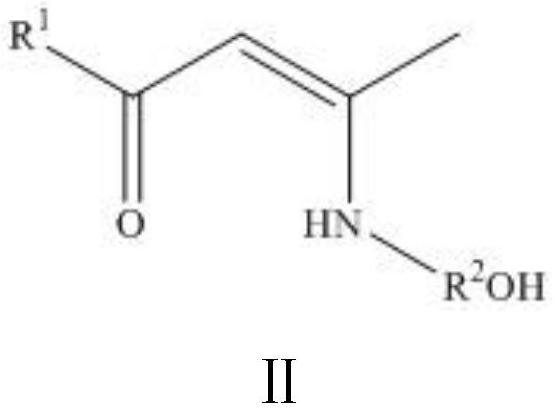
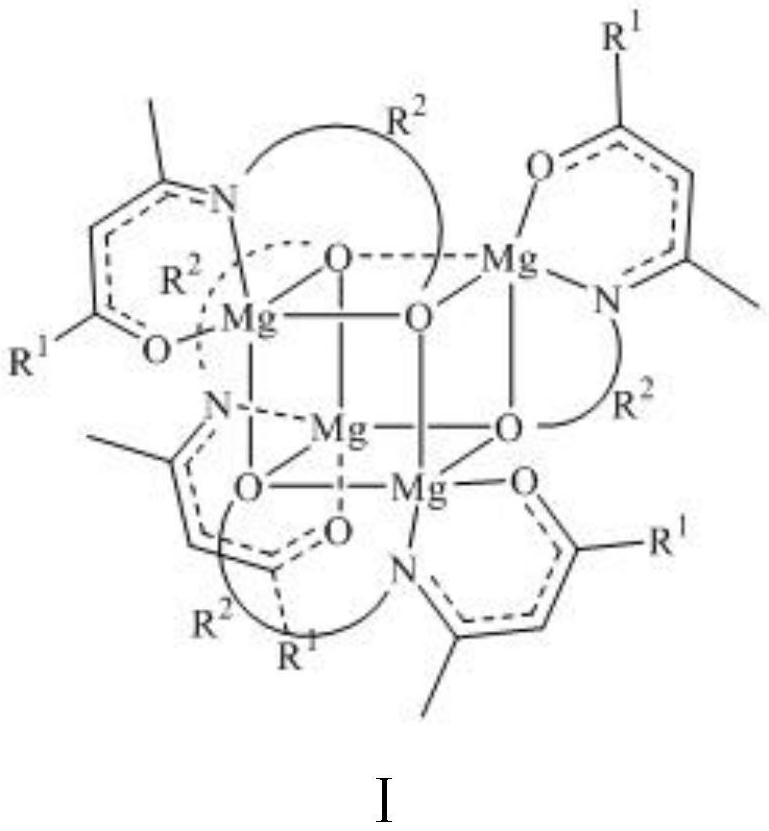
[0025] [CH 3 C(O)CHCN(CH 2 CH 2 O)CH 3 ] 4 Mg 4 Synthesis of (2a): In an argon atmosphere, take 1a (0.45 g, 3.1 mmol) and dissolve it in anhydrous and oxygen-free tetrahydrofuran, place the reaction in an ice-water bath at 0°C, and add dibutylmagnesium dropwise (2.5 mL, 1 M in n-hexane, 2.5 mmol), the reaction was stirred at 0 °C for 3 h to give a pale yellow clear solution. The solvent was dried in vacuum, washed three times with 6 mL of n-hexane to obtain a pale yellow powder, and the product was characterized by nuclear magnetic resonance and elemental analysis. Yield: 0.68 g (33%). 1 H-NMR (400MHz, CDCl 3 ): δ=4.71(s, 4H, [OC(C-H)CN]), 3.60–3.56(m, 4H, NCH 2 CH 2 ), 3.44 (br, 4H, NCH 2 CH 2 ), 3.25-3.22 (m, 4H, NCH 2 CH 2 ), 3.04-2.97 (m, 4H, NCH 2 CH 2 ), 1.84(s, 12H, CH 3 CO), 1.78 (s, 12H, CH 3 EN). 13 C-NMR (400MHz, CDCl 3 ): δ=179.06(C=O), 170.71(C-N), 96.82([OC(C-H)CN]), 61.36(CH 2 O), 51.46 (NCH 2 ), 27.41 (CH 3 CO), 21.30 (NCCH 3 ).Anal.Calcd...
Embodiment 2
[0027] [CH 3 C(O)CHCN(CH 2 CH 2 CH 2 O)CH 3 ] 4 Mg 4 Synthesis of (2b): In an argon atmosphere, 1b (0.73 g, 4.6 mmol) was dissolved in tetrahydrofuran treated with anhydrous and anoxic, and dibutylmagnesium (3.7 mL, 1 M inn- hexane, 3.7 mmol) and the reaction was stirred at 55 °C overnight to give a milky cloudy solution. The reaction was cooled to room temperature, the layers were statically separated, filtered to obtain a yellow filtrate, the solvent was vacuum dried, washed three times with 6 mL of n-hexane to obtain a yellow powder, and the product 2b was characterized by NMR. 1 H-NMR (400MHz, CDCl 3 ): δ=4.66(s, 4H, [OC(C-H)CN]), 3.53–3.49(m, 8H, NCH 2 CH 2 CH 2 ), 3.42-3.31 (m, 8H, NCH 2 CH 2 CH 2 ), 1.83(s, 12H, CH 3 CO), 1.81 (s, 12H, CH 3 CN), 1.52-1.45 (m, 8H, NCH 2 CH 2 CH 2 ).
Embodiment 3
[0029] [CF 3 C(O)CHCN(CH 2 CH 2 O)CH 3 ] 4 Mg 4 Synthesis of (2c): In an argon atmosphere, 1c (0.56 g, 2.8 mmol) was dissolved in anhydrous and oxygen-free tetrahydrofuran, the reaction was placed in an ice-water bath at 0°C, and dibutylmagnesium was added dropwise (2.3 mL, 1 M in n-hexane, 2.3 mmol), the reaction was stirred at 0 °C for 3 h to give a clear, colorless solution. The solvent was vacuumed to dryness, washed three times with 6 mL of n-hexane to obtain a white powder, and the product 2c was characterized by NMR. 1 H-NMR (400MHz, CDCl 3 ): δ=5.25(s, 4H, [OC(C-H)CN]), 3.59-3.54(m, 4H, NCH 2 CH 2 ), 3.46-3.40 (m, 4H, NCH 2 CH2 ), 3.38-3.36 (m, 4H, NCH 2 CH 2 ), 3.09-3.02 (m, 4H, NCH 2 CH 2 ), 1.93(s, 12H, CH 3 EN).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com