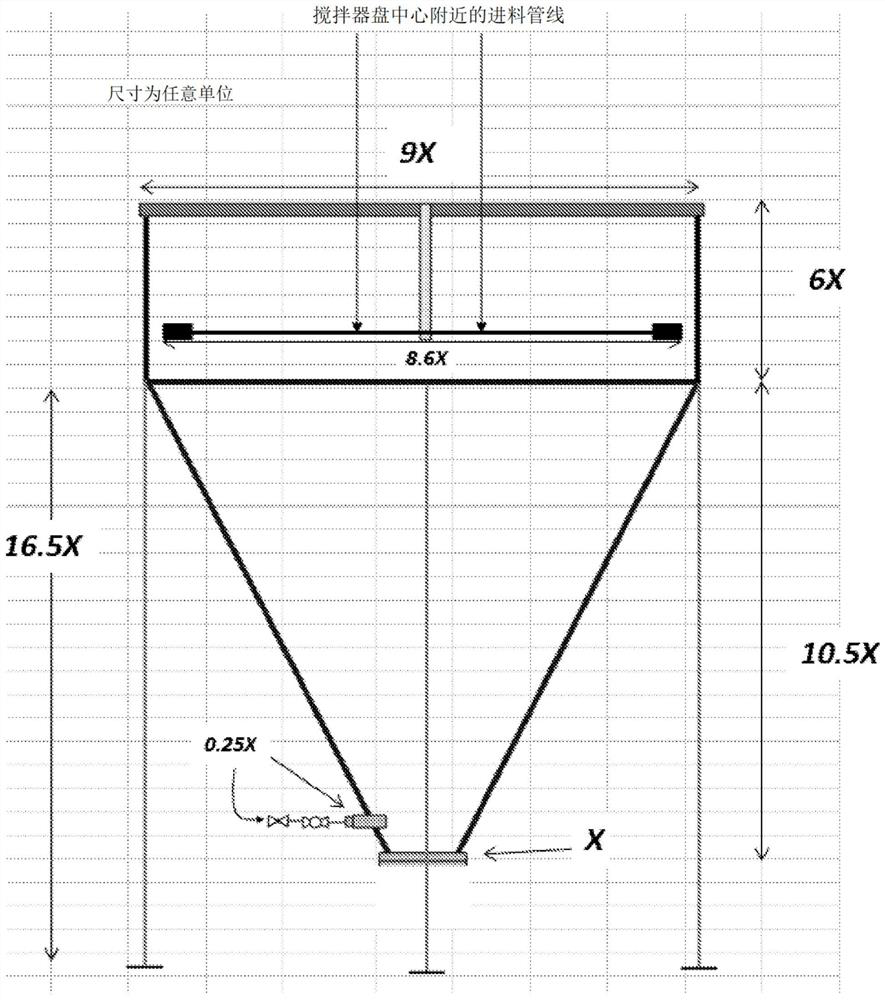
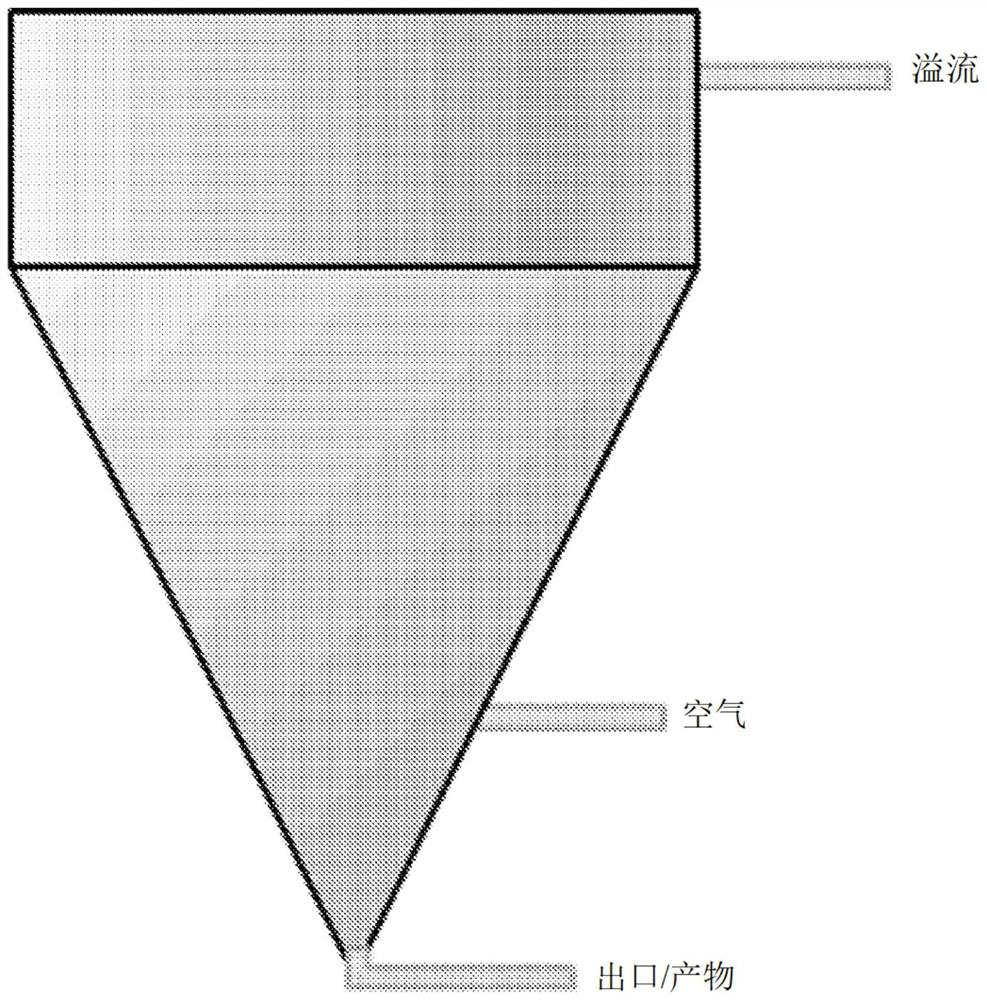
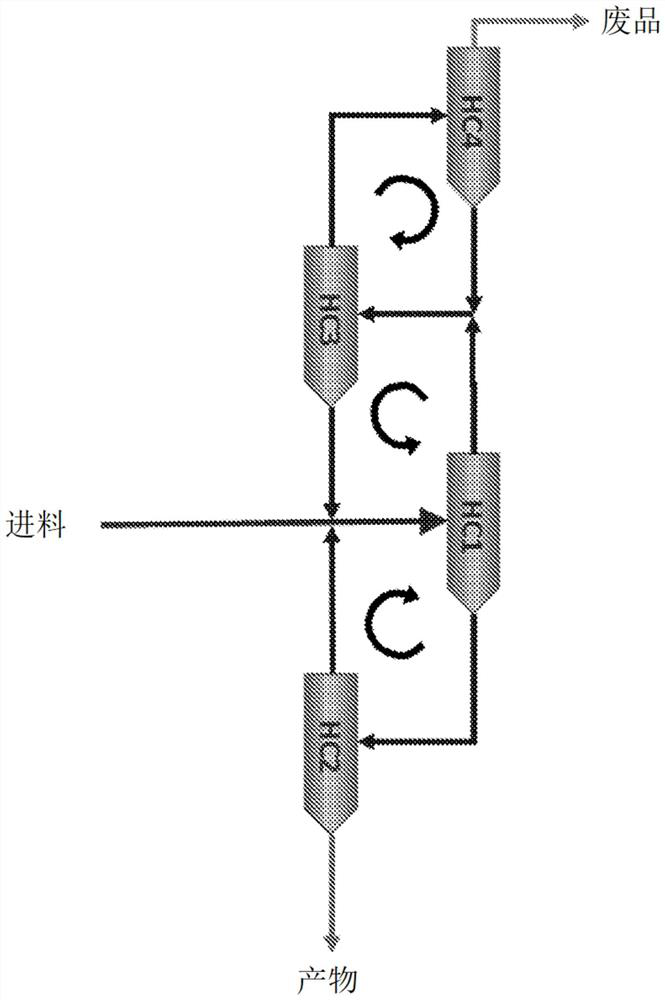
Method for producing purified precipitated calcium carbonate from lime sludge
A technology for precipitating calcium carbonate and limestone, applied in chemical instruments and methods, calcium carbonate/strontium/barium, lime production, etc., can solve the problems of increasing the specific surface area of lime mud particles and being unsuitable
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Heat aging (HA) in sodium carbonate
[0085] The lime mud is treated to provide a precipitated calcium carbonate material with reduced specific surface area and reduced impurity content compared to the starting material. The lime mud cake is obtained as a waste product from the pulp mill. The lime mud cake is slurried in a 25%-30% sodium carbonate solution with a calcium carbonate solid content of 10%-20%. In particular, in 1 kg of slurry, 200 g of CaCO 3 (20%CaCO 3 ), 240gNa 2 CO 3 , 560g H 2 O(30%Na 2 CO 3 ).
[0086] The slurry was then heat aged at 100°C for 8 hours to form a mixed salt, calcium hydroxide (Na 2 Ca(CO 3 ) 2 ·5H 2 O). Methods without the heat aging step were compared and the results are presented in the table below.
[0087] The slurry was then filtered or separated into solid and liquid phases by vacuum filtration using a Buchner funnel and filter paper. The filter cake was washed in a Buchner funnel with three portions of w...
Embodiment 2
[0093] Example 2: Removal of impurities using phase separation
[0094] The lime mud cake obtained as waste from the pulp mill was slurried at 20% solids in water. The pulp is processed using a filter press with a wash cycle to remove excess white liquor from the pulp mill. A filter press was used to first form a filter cake from the 20% solids slurry and then force water through the filter cake. The wash cycle was performed with an amount of water four times the mass of dry solids.
[0095] The washed filter cake was dispersed in water at 20% solids using sodium poly(acrylate) as the chemical dispersant. The resulting dispersed slurry had a viscosity of about 10 cps at 100 rpm.
[0096] The dispersed slurry was then aerated with flue gas having a carbon dioxide content of about 15% to lower the pH to a pH of about 10.5.
[0097] The pH adjusted slurry is then ground to the desired particle size suitable for use as a paper filler or coating pigment. In this example, the p...
Embodiment 3
[0101] Example 3: Removal of impurities using phase separation of lime mud prior to grinding
[0102]The lime mud cake obtained as waste from the pulp mill was slurried at 20% solids in water. The slurry was processed using a horizontal filter press with a wash cycle to remove excess white liquor in the liquid phase. A filter press was used to first form a filter cake from a 20% solids slurry, resulting in a 65% solids filter cake, and then water was forced through the filter cake. The wash cycle was performed with an amount of water four times the mass of dry solids.
[0103] The resulting washed filter cake was slurried in water at 23% solids and then treated by aeration with carbon dioxide-containing flue gas to lower the pH to 10.9.
[0104] The pH adjusted slurry was then continuously centrifuged at 1300 rpm to obtain a g-force of 615, with residence times varying as indicated in the table. The centrifuge filtrate containing fine particulate impurities was removed and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com