Application of heptamethine indocyanine small molecules with N-alkyl side chains with different chain lengths in preparation of photosensitizer for treating tumors
An indocyanine and small molecule technology, applied in the field of biomedical engineering, can solve the problems of remote metastatic tumor immunotherapy research, unsatisfactory phototherapy effect, and practical application limitations without phototherapy to activate the body's systemic anti-tumor immune response. , to amplify the immunogenic death of tumor cells, inhibit tumor growth, and enhance the effect of phototherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
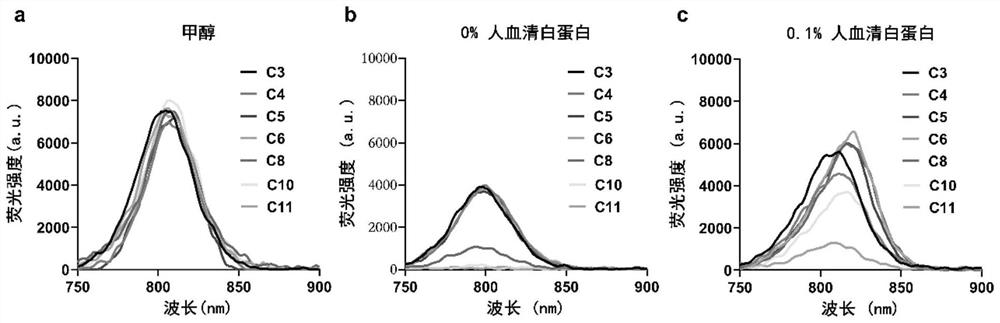
[0034] Example 1 Spectroscopic determination of small molecules C3-C11 of heptacyanine with different chain lengths
[0035] Accurately weigh the small molecules C3-C11 of heptamethine indocyanine with different chain lengths with a 1 / 10,000 analytical balance, and prepare a 10 mM dimethyl sulfoxide (DMSO) solution for later use. During the test, the dye solution was diluted to 4 μM with DMSO, and the UV absorption spectrum, fluorescence emission spectrum and fluorescence quantum yield (Φ) of compounds C3-C11 were measured by ShimadzuUV-3600 UV-Fluorescence Spectrophotometer and Lumina Fluorescence Spectrometer respectively. F). As shown in Table 1, the test results show that the maximum ultraviolet absorption wavelength and maximum fluorescence emission wavelength of all compounds are located in the near-infrared region (700-900nm), and the molar absorption coefficient (ε) is in the range of 100 000-350 000, showing excellent Light Harvesting Capability and Fluorescence Emis...
Embodiment 2
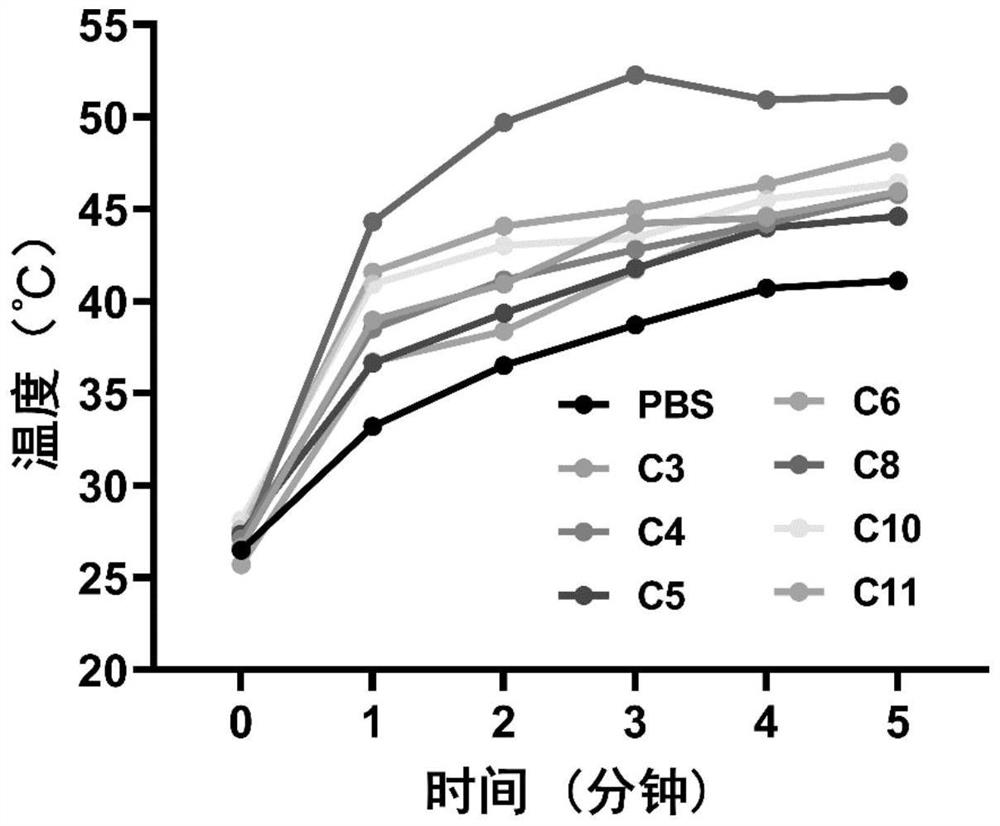
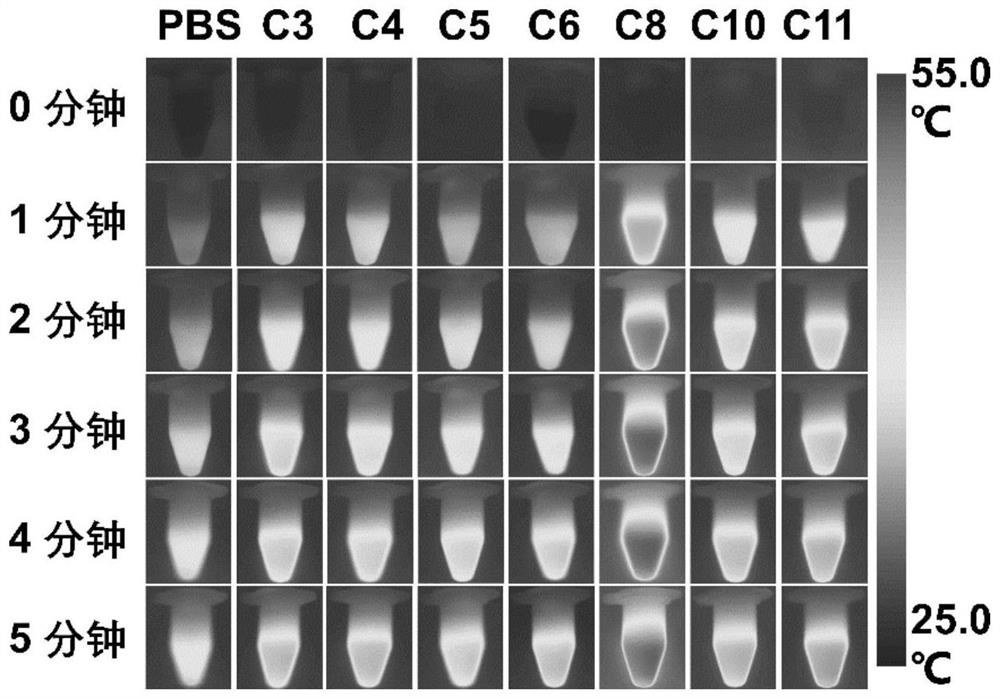
[0041] Example 2 Photothermal effects of small molecules C3-C11 of heptacyanine with different chain lengths
[0042] Compounds C3 to C11 were prepared as 10 mM stock solutions with DMSO and stored at -20°C. A certain amount of stock solution was drawn from the stock solutions of C3-C11 compounds prepared above and added to phosphate buffered saline (PBS, 10mM, PH=7.4) containing 1% HSA to prepare a 10 μM sample working solution, and 1.5 mL of each was added. in a centrifuge tube. Place the centrifuge tube containing the detection solution at 808nm, 1.0W / cm 2 The near-infrared laser (Laser) was irradiated for 5 minutes, and the temperature change was recorded every 30 seconds. The results are shown in figure 2 and image 3 . The experimental results show that compounds C3-C11 have the effect of inducing temperature rise, among which the temperature rise effect of C8 is the most excellent, which can reach above 55℃.
Embodiment 3
[0043] Example 3 Singlet Oxygen Generation of Heptamethanine Small Molecules C3~C11 with Different Chain Lengths
[0044] SOSG probes (prepared in 100% methanol solution) were added to 2 mL of 10 μM aqueous solution of heptamethanine small molecules with different chain lengths, so that the SOSG concentration was 1.5 μM and the methanol content was 2%. and then at 1W / cm 2 The 808 nm laser was irradiated for 5 minutes, followed by excitation at 495 nm, and the fluorescence emission spectrum at 500-600 nm was immediately measured, and the generation of singlet oxygen was quantified by the fluorescence intensity of the maximum emission peak at 525 nm. like Figure 4 As shown, C3-C11 can generate a large amount of singlet oxygen after being irradiated by near-infrared 808 nm laser, among which compound C8 has the strongest singlet oxygen generation ability, which provides feasibility for realizing tumor photodynamic therapy.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com