Galanthamine hydrobromide cream and preparation method thereof
A technology of galantamine hydrobromide and ointment, applied in ointment delivery, pharmaceutical formula, aerosol delivery, etc., can solve problems such as poor drug compliance and difficulty swallowing, achieve high encapsulation rate and simple preparation process , Great skin permeability and stability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 galantamine hydrobromide cream (1) preparation of galantamine hydrobromide liposome
[0039] 1. Experimental materials
[0040] 1.1 Reagents Galantamine hydrobromide (Zhejiang Yixin Pharmaceutical Co., Ltd.), lecithin (Beijing Aoxing Biotechnology Co., Ltd.), cholesterol (BR Shanghai Zhengxiang Institute of Chemical Reagents).
[0041] 1.2 Instruments RE-524 Rotary Evaporator (Shanghai Yarong Biochemical); KT-300Y Ultrasonic Drug Processor (Jining Zhongguan Ultrasonic Co., Ltd.); XSZ-109 Optical Microscope (Guangzhou Instrument Factory); 752C UV-Vis Spectrophotometer Meter (Shanghai Third Instrument Factory).
[0042] 2. Methods and Results
[0043] 2.1 Preparation (1) Preparation of phosphate buffer solution (pH 5.0) Take a certain amount of 0.2mol / L sodium dihydrogen phosphate solution, and adjust the pH value to 5.0 with sodium hydroxide test solution.
[0044] (2) Thin film-ultrasonic method (TFV) Weigh an appropriate amount of lec...
Embodiment 2
[0062] The preparation of embodiment 2 Galantamine hydrobromide cream
[0063] (1) Preparation of Galantamine Hydrobromide Liposomes
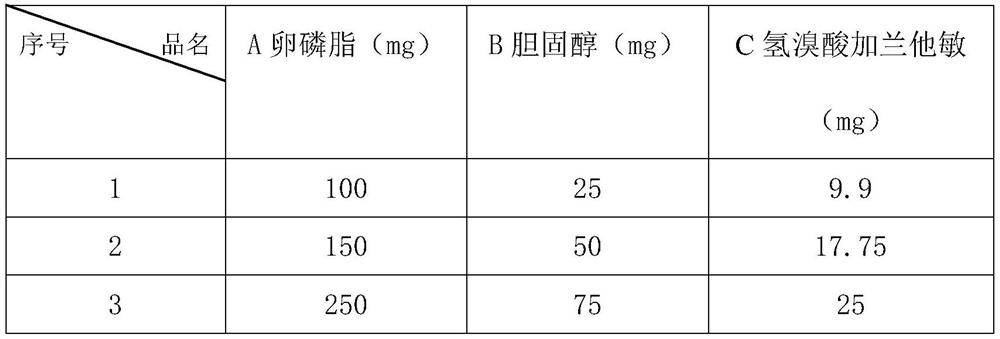
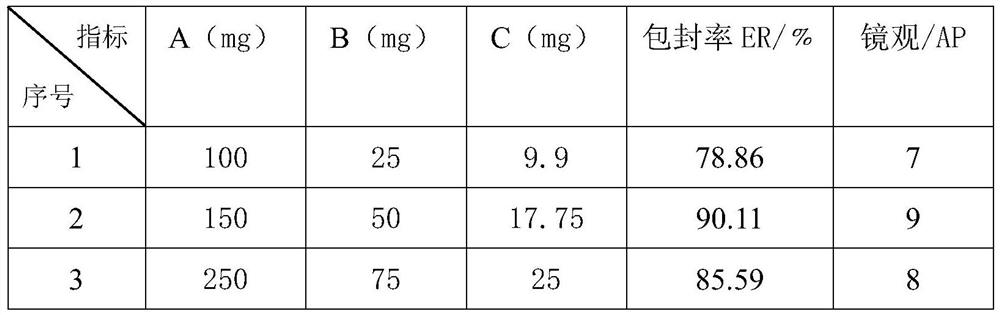
[0064] Lecithin 210mg cholesterol 70mg Galantamine hydrobromide 20mg
[0065] Preparation method: Thin film-ultrasonic method (TFV) Weigh the prescription amounts of lecithin, cholesterol and galantamine hydrobromide, add appropriate amount of ethanol to dissolve them, put them in a pear-shaped bottle, and rotate under reduced pressure to evaporate the lipids in the bottle. The inner wall is uniformly formed into a film, drained, and an appropriate amount of phosphate buffer (pH 5.0) is added, shaken to transfer the lipid film from the bottle wall into the water phase, fully swelled with water, sonicated for 10 minutes, and then adjusted to volume with phosphate buffer. 20ml is ready.
[0066] (2) Preparation of cream base
[0067]
[0068]
[0069] The preparation method is the same as in Example 1.
[0...
Embodiment 3
[0072] The preparation of embodiment 3 Galantamine hydrobromide cream
[0073] (1) Preparation of galantamine hydrobromide liposomes
[0074] Lecithin 390mg cholesterol 130mg Galantamine hydrobromide 45mg
[0075] The preparation method is the same as that of Example 2.
[0076] (2) Preparation of cream base
[0077] Glyceryl Monostearate 20g octadecanol 20g Propylene Glycol 10g methylparaben 0.2g Propylparaben 0.1g Sodium dodecyl sulfate 0.5g
[0078] The preparation method is the same as in Example 1.
[0079] (3) Preparation of cream
[0080] The preparation method is the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com