Diaryl acetylene compound and preparation method thereof
A technology for diarylacetylenes and compounds, which is applied in the field of diarylacetylenes and their preparation, can solve the problems of difficulty in controlling the singleness of products, unfavorable long-term storage, unfavorable purification and purification, etc., and achieves a wide range of substrate expansion, The post-processing is simple, efficient and environmentally friendly, and the preparation method is simple and convenient.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048]
[0049] The reaction formula of the preparation method of the diarylacetylene compound of the present invention is:
[0050]
[0051] where R 1 and R 2 All are selected from one or more of chlorine, bromine, fluorine, trifluoromethyl, methyl, ethyl, nitro, aldehyde, methoxy, hydrogen, pyridyl, hydroxyl or methyl carboxylate, R 1 , R 2 Can be the same or different.
[0052] Specifically include the following steps:
[0053](1), in DMF, successively add iodoaromatic compounds, trimethylsilyl acetylene, metal catalyst and alkali lye, stir; Then add DBU, water and iodoaromatic compounds successively, raise temperature, continue to stir, get the mixture;
[0054] (2), after the reaction finishes, the mixed solution is filtered through diatomaceous earth to remove unnecessary metals, under stirring, add water and continue to stir, after a large amount of solids are separated out, filter, and the filter cake is washed with a large amount of water to remove residual ...
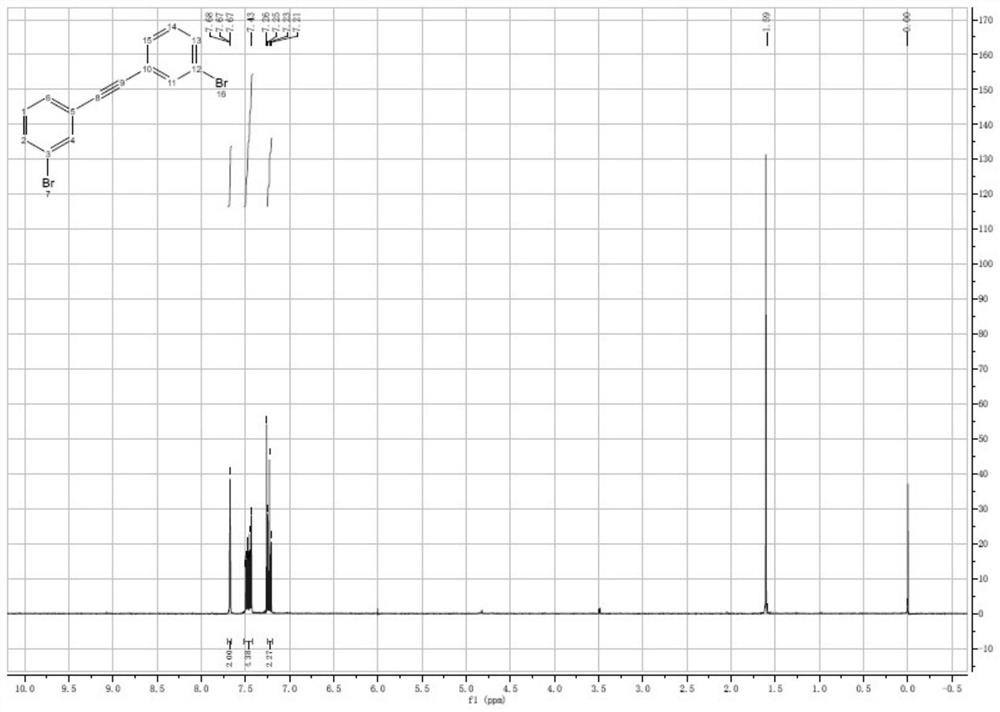
Embodiment 1
[0074] The preparation method of 1,2-bis(3-bromophenyl)acetylene of the present embodiment comprises the following steps:
[0075] (1), weigh 11.0g of 3-bromoiodobenzene into a 500mL three-necked flask, add 110mL of DMF, then add 4.21g of Trimethyl silyl acetylene, 0.44g of CuI, 0.39g of PdCl 2 (PPh 3 ) 2 and 14.25g of Et 3 N, nitrogen was replaced three times, and stirred at 60 °C for 3 h. Subsequently, 35.6 g of DBU, 0.28 g of deionized water and 11.0 g of 3-bromoiodobenzene were added in sequence, the temperature was raised to 80° C., and stirring was continued for 3 h.
[0076] (2), after the LC-MS monitoring reaction finishes, remove excess metal through diatomaceous earth filtration, under stirring, add twice the volume of water, continue stirring for 15min, after seeing a large amount of solid precipitation, filter, filter cake with After washing with a large amount of water to remove the residual solvent, the solid was vacuum-dried for 2 hours to obtain 10.0 g of ...
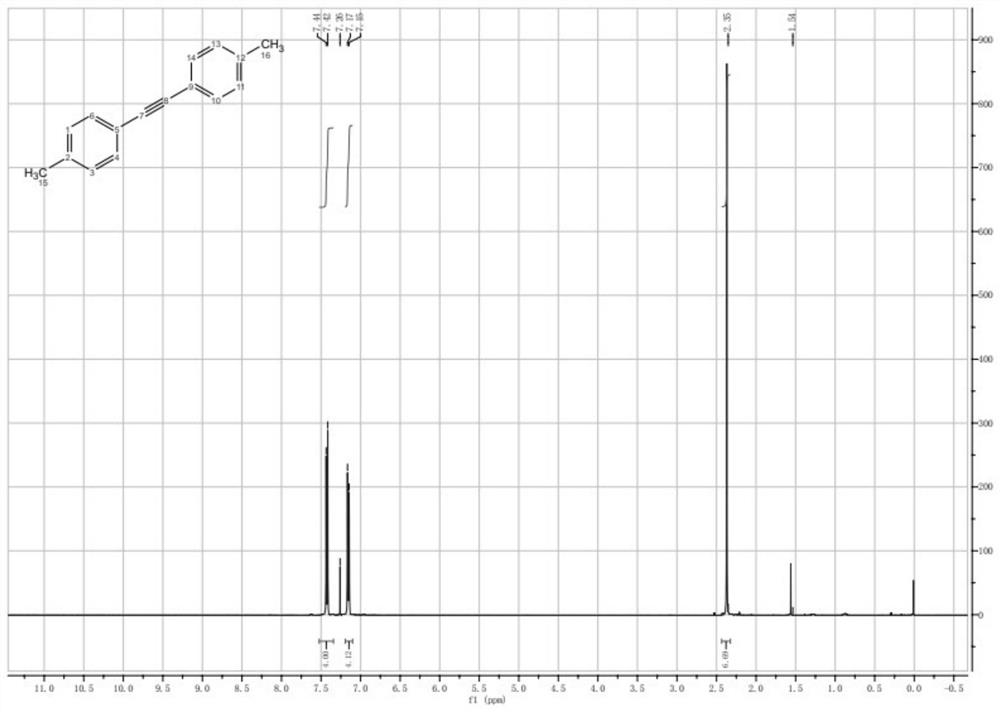
Embodiment 2
[0083] The preparation method of 1,2-bis(4-methylbenzene)acetylene of the present embodiment comprises the following steps:
[0084] (1), weigh 8.48g of 4-methyl iodobenzene into a 500mL three-necked flask, add 110mL of DMF, then add 4.21g of Trimethyl silyl acetylene, 0.44g of CuI, 0.39g of PdCl 2 (PPh 3 ) 2 and 14.25g of Et 3 N, nitrogen was replaced three times, and stirred at 60 °C for 3 h. Subsequently, 35.6 g of DBU, 0.28 g of deionized water and 8.48 g of 4-methyl iodobenzene were added in sequence, the temperature was raised to 80° C., and stirring was continued for 3 h.
[0085] (2), after the LC-MS monitoring reaction finishes, remove excess metal through diatomaceous earth filtration, under stirring state, add twice the volume of water, continue stirring for 10min, after seeing a large amount of solid precipitation, filter, filter cake with Wash with a lot of water to remove the residual solvent, and the solid is vacuum-dried for 1 h to obtain 5.8 g of the desi...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap