Method for preparing iron phosphate through two-step hydrochloric acid dissolution of pyrite cinders
A technology of pyrite slag and iron phosphate, applied in the field of comprehensive utilization of solid waste resources, can solve the problems of low utilization rate, meet equipment requirements and low energy consumption, reduce environmental pollution, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
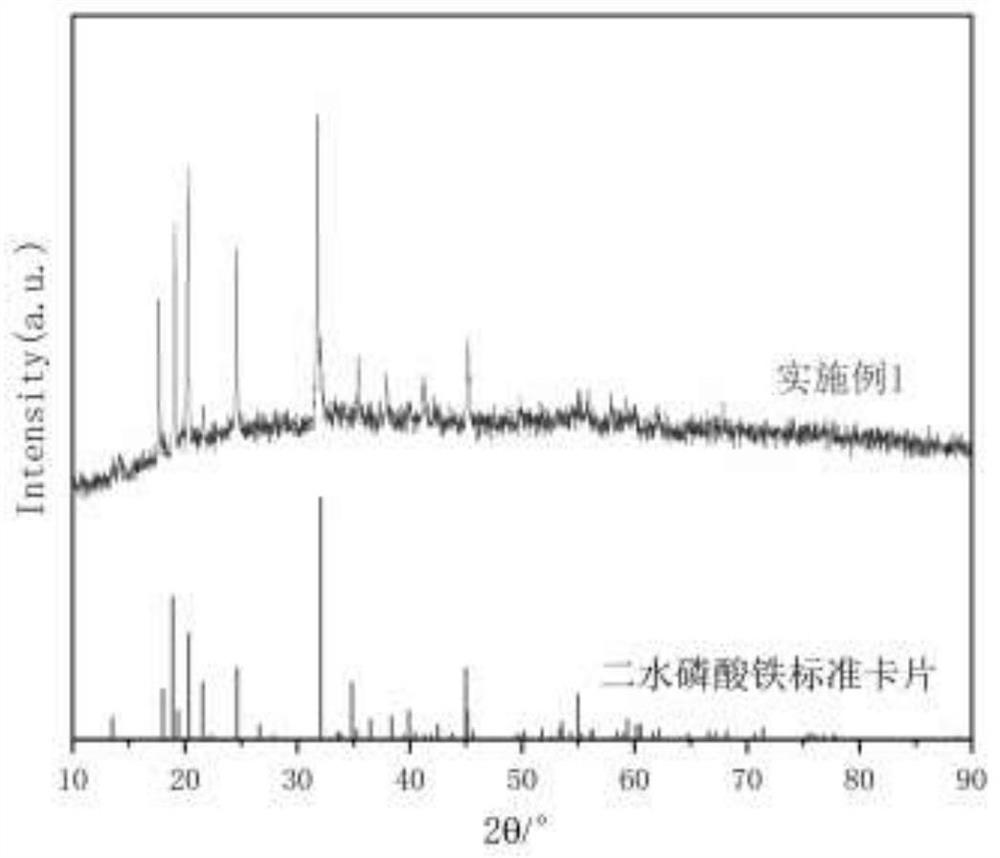
Embodiment 1
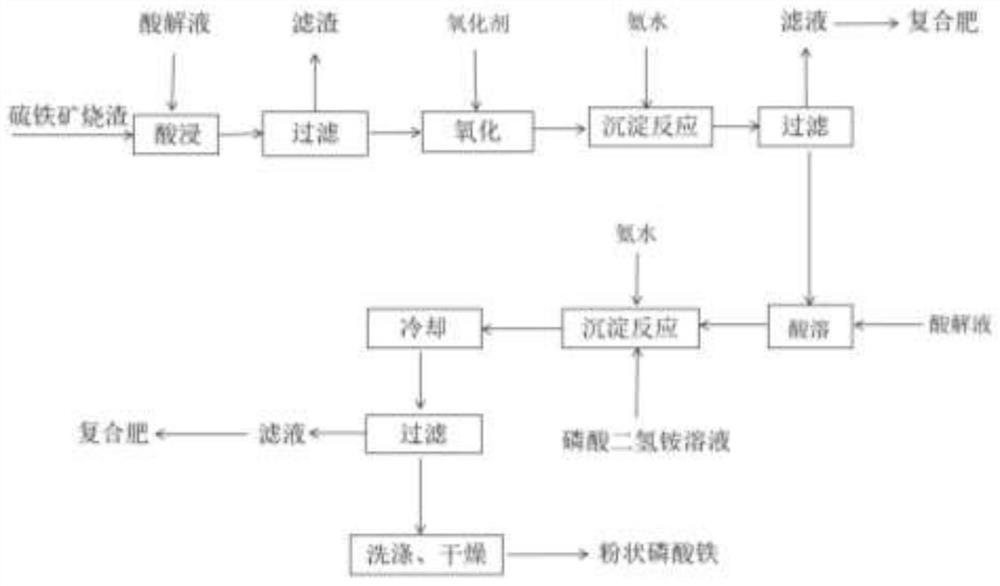
[0033] A method for preparing iron phosphate by two-step acid dissolving of pyrite slag by hydrochloric acid method, and its process flow diagram is shown in figure 1 The pyrite slag adopted is the product after high temperature calcination in a factory with pyrite for acid production, and its main component content (mass percentage) is as follows: the iron content (calculated as iron oxide) is 59.81%, the silicon content ( with SiO 2 Calcium (as MgO) 5.44%, magnesium content (as MgO) 2.61%, calcium content (as CaO) 1.91%, sulfur (as sulfate) 1.69%, aluminum content (as Al 2 O 3 Calculation) is 0.85%; it specifically includes the following steps:
[0034] 1) pretreatment: the pyrite slag is washed with water, filtered, dried and ground to obtain slag powder;
[0035] 2) Acid leaching: take 80.00 g of cinder powder and add a hydrochloric acid solution with a concentration of 37 wt %, control the quality of the HCl introduced into it to be 1.4 times the total iron content of ...
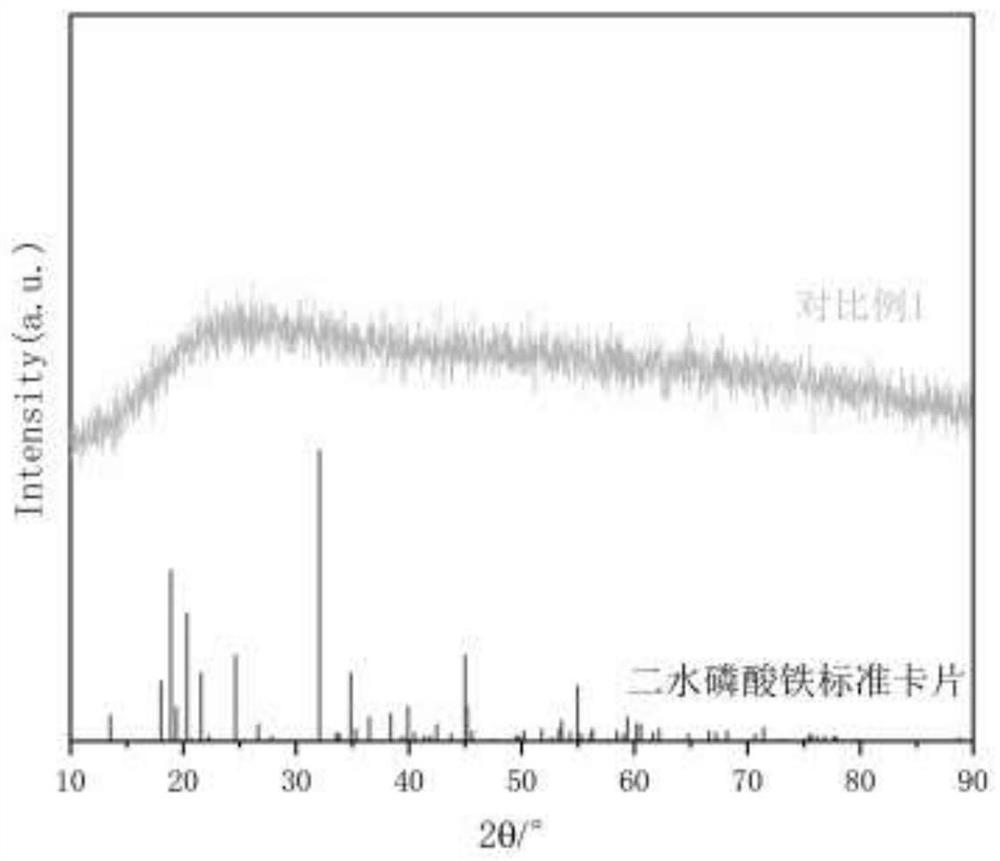
Embodiment 2
[0041] A method for preparing iron phosphate by two-step acid dissolving of pyrite slag by hydrochloric acid method. The content (as iron oxide) is 58.81%, the silicon content (as SiO 2 Calcium (as MgO) 5.40%, magnesium content (as MgO) 2.60%, calcium content (as CaO) 1.91%, sulfur (as sulfate) 1.69%, aluminum content (as Al 2 O 3 Calculation) is 0.85%; it specifically includes the following steps:
[0042] 1) pretreatment: the pyrite slag is washed with water, filtered, dried and ground to obtain slag powder;
[0043] 2) Acid leaching: take 80.00 g of slag powder and add a hydrochloric acid solution with a concentration of 37wt%, control the quality of the HCl introduced into it to be 1.4 times the total iron content of the slag powder, pickle at 90°C for 4 hours, and the iron leaching The rate reached 89.32%;
[0044] 3) Acid leaching solution purification: the obtained acid leaching solution is treated with H 2 O 2 The solution (30wt%) was oxidized at room temperature...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com