Application of saccharomyces cerevisiae in catalyzing citral to generate dextrorotatory citronellal and method for preparing levorotatory isopulegol
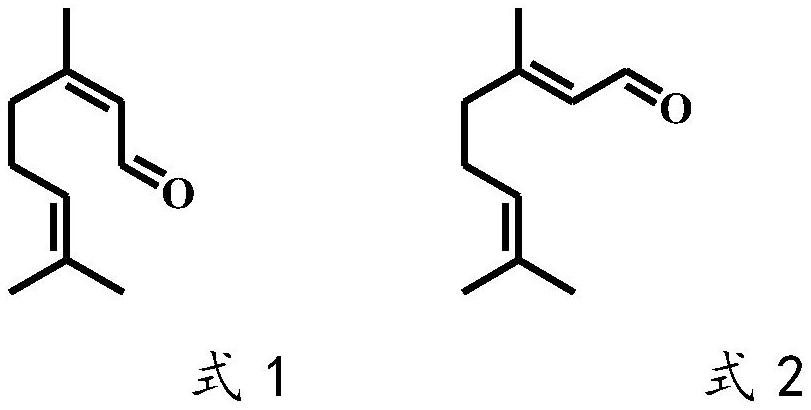
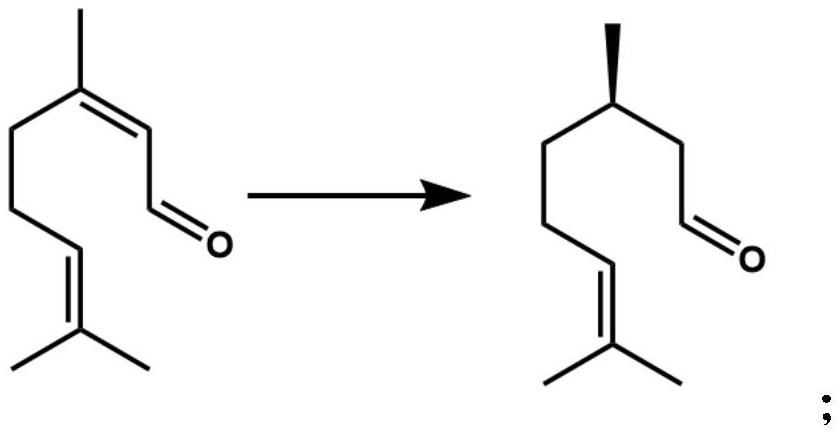
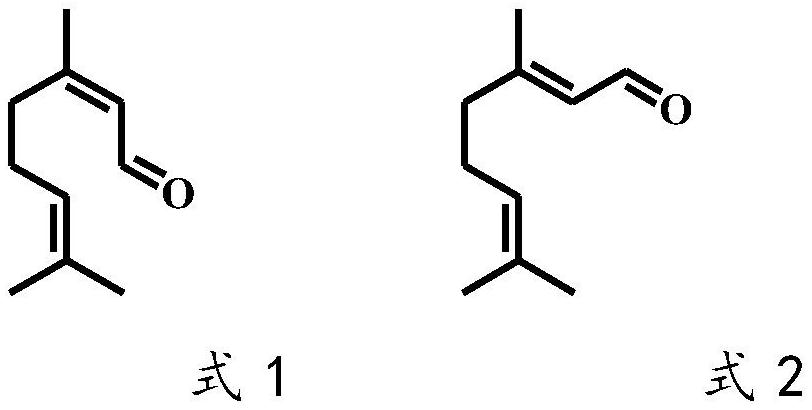
A technology of isopulegol and Lithospora, which is applied to the application of Trichosporidium lubilis in selectively catalyzing citral to generate dextro-citronellal, and the field of preparing levo-isopulegol, which can solve the problem of neral aldehyde The problems of low stereoselectivity and low optical purity of products can achieve the effect of good industrial application prospects, good optical purity and high stereoselectivity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Preparation of T. lubilis CGMCC 1292 wet cells
[0044] Seed liquid medium formula: glucose 1%, peptone 0.5%, beef extract 0.8%, pH 7.0. High temperature sterilization at 121°C for 20min.
[0045] Fermentation medium formula: glucose 15g / L, beef extract 8g / L, peptone 5g / L, KH 2 PO 4 2.0g / L, Na 2 HPO 4 2.0g / L, NaCl 0.5g / L, MgSO 4 0.5g / L, pH 7.0. Sterilize at 121℃ for 20min.
[0046] Take the slant of the yeast CGMCC 1292 stored at 4°C, pick a loop and inoculate it into a 250 mL shake flask containing 50 mL of seed liquid medium. Incubate for 24h at 30°C, 200rpm shaker, transfer to a 1L shake flask with 300mL fermentation medium at 5% (v / v) inoculum, continue to culture at 30°C, 200rpm for 36h, and harvest wet cells by centrifugation .
Embodiment 2
[0047] Example 2 Preparation of the crude enzyme powder of Rhizopus bilis CGMCC 1292
[0048] 100 g of cells harvested in Example 1 were weighed, suspended in 1 L of Tris-HCl buffer (100 mM, pH 8.0), and cooled in an ice-water bath. The cell suspension was disrupted twice using a high pressure homogenizer at 1800 bar. The disrupted liquid was centrifuged at 4°C and 15000 rpm for 10 min to obtain a clear centrifuged supernatant, which was a cell-free extract. The cell-free extract was quickly frozen in a -80°C refrigerator, and then freeze-dried using a freeze dryer to obtain freeze-dried crude enzyme powder.
Embodiment 3
[0049] Example 3 Determination of Stereoselectivity Catalyzed by Trichosporidium licheniformis citral olefinic reductase
[0050] In a 2 mL Eppendorf tube, add 100 μL of the cell-free extract as described in Example 2, 10 μL of neral or geranial in methanol (100 mM), 20 μL of NADPH in water (100 mM), and 870 μL of Tris-HCl buffer (100 mM) , pH 8.0), placed on a shaking mixer, 30 ° C, 1000 rpm shaking reaction for 2 h. Extract three times with equal volume of ethyl acetate, add anhydrous sodium sulfate to the extract and dry, and detect the stereo configuration and optical purity of the product citronellal by gas chromatography with a chiral chromatographic column.
[0051] The gas chromatography column used is a BGB-174 chiral chromatography column, 30m×0.25mm×0.25μm, the temperature of the injector and the detector is 250°C, the initial column temperature is 40°C, and the temperature is increased to 120°C at a rate of 4°C / min , maintained for 1 min, and then heated to 180 °C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com