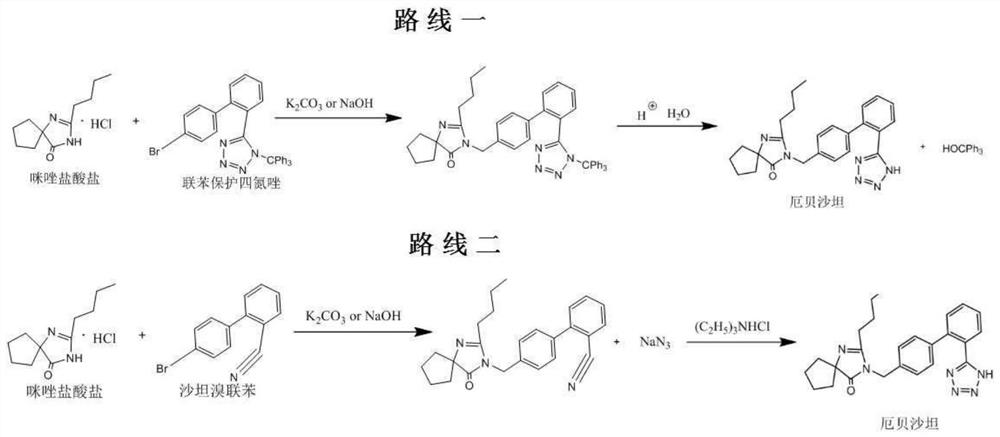
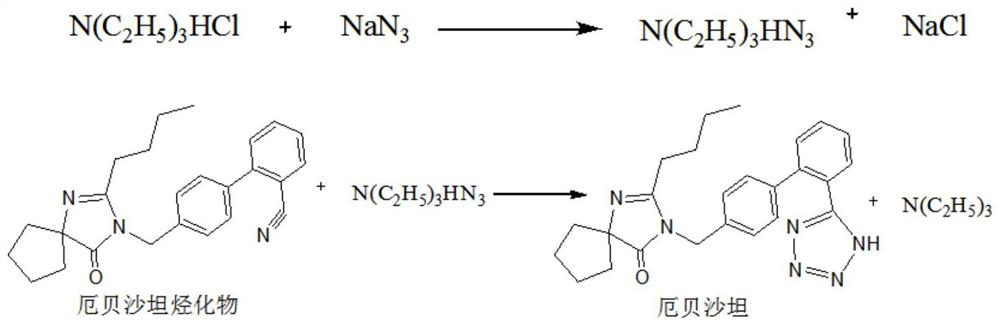
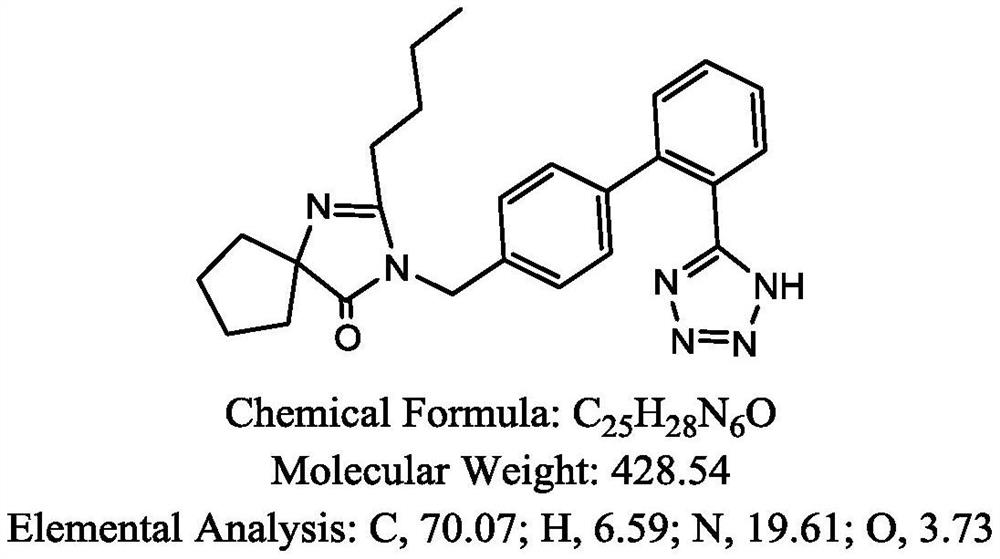
Method for preparing irbesartan without solvent
A solvent-free and solution-free technology, applied in organic chemistry, bulk chemical production, etc., can solve the problems of increased difficulty in product drying and desolvation, increased difficulty in three wastes treatment, increased post-treatment procedures, etc., to achieve safe and reliable reaction conditions, improve Purity and quality stability, the effect of reducing the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] 1) Preparation process: in a 2000mL four-necked reaction flask, add irbesartan hydrocarbyl compound, namely 4'-[(2-butyl-4-oxo-1,3-diazaspiro[4.4]nonane-1) -Alken-3-yl)methyl]biphenyl-2-carbonitrile 300g (0.778mol), triethylamine hydrochloride 107.0g (0.778mol), heated to 80 ℃, the solid material began to melt, when the raw material was completely When melted into a liquid state, start stirring, add sodium azide in 3 times, add 17.2g each time, and add 51.6g (0.778mol) in total at an interval of 1h, then continue to stir and heat up to 100 ° C, keep the reaction 24h, the reaction solution was obtained.
[0040] 2) Post-treatment: prepare 10% liquid caustic soda (sodium hydroxide 77.8 g+water 700 mL), add the liquid caustic soda to the reaction solution in the reaction flask, stir for 30 min to dissolve the feed liquid, then transfer to a separatory funnel, and stand for stratification 1h, the feed liquid is divided into upper and lower layers, the lower alkaline salt-c...
Embodiment 2
[0043] 1) preparation process: in 2000mL four-necked reaction flask, add irbesartan hydrocarbyl compound 300g (0.778mol), triethylamine hydrochloride 160.0g (1.167mol), be heated to be warming up to 100 ℃ to raw material and melt into liquid state completely, Then start stirring, continue to stir and heat up to 110 ° C, and add 75.9 g (1.167 mol) of sodium azide in 5 times, adding 15.18 g each time, each time interval is 1 h, and keep reacting for 20 h to obtain a reaction solution.
[0044] 2) Post-processing: prepare 15% liquid caustic soda (sodium hydroxide 120 g+water 680 mL), add the liquid caustic soda to the reaction solution in the reaction flask, stir for 30 min to dissolve the feed liquid; transfer to a separatory funnel, and let stand for stratification for 1 hour , the feed liquid is divided into upper and lower layers, and the lower alkaline salt-containing phase is discarded; the organic phase of the upper product layer (mainly composed of triethylamine and the pr...
Embodiment 3
[0047] 1) preparation process: in 2000mL four-hole reaction flask, add irbesartan hydrocarbyl compound 300g (0.778mol), triethylamine hydrochloride 214.0g (1.556mol), be heated to be warming up to 100 ℃ to raw material and melt into liquid state completely, Then start stirring, continue to stir and heat up to 110 ° C, and add 101.2 g (1.556 mol) of sodium azide in 3 times, adding 33.73 g each time, each time interval is 1 h, and keep reacting for 18 h to obtain a reaction solution.
[0048] 2) Post-treatment: prepare 20% liquid caustic soda (sodium hydroxide 160 g+water 640 mL), add the liquid caustic soda to the reaction solution in the reaction flask, and stir for 30 min to dissolve the feed solution. Transfer to a separatory funnel, let stand for stratification for 1 hour, the feed liquid is divided into upper and lower layers, discard the lower alkaline brine-containing phase; the upper product layer organic phase (mainly composed of triethylamine and product) is transferre...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com