Synthesis method of 2-cyano-2-oximido acetamide sodium salt and cymoxanil
An oxime-acetamide sodium salt and a synthesis method technology, applied in the field of pesticide synthesis, can solve the problems of large amount of three wastes, high cost of raw materials, low reaction yield, etc., to reduce the pressure of environmental protection treatment, easy to recycle, high product yield rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
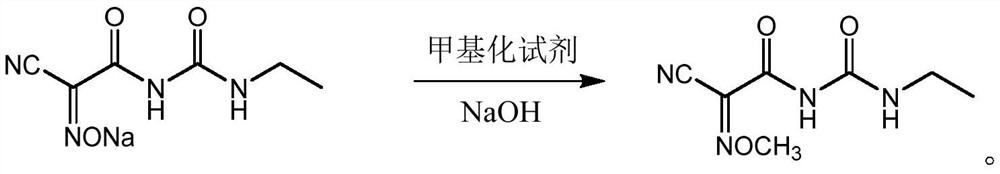
[0033] The method for synthesizing the trioxuron comprises the following steps: carrying out a methylation reaction with the 2-cyano-2-oximoacetamide sodium salt reaction solution obtained by the oximation reaction, a methylating reagent and an alkali solution, and after the reaction is completed, Cooling and crystallization.
[0034] For reference, the alkaline solution includes aqueous sodium hydroxide solution, aqueous potassium hydroxide solution, aqueous sodium carbonate solution or aqueous triethylamine solution. In a preferred embodiment, the alkaline solution is an aqueous sodium hydroxide solution.
Embodiment 1
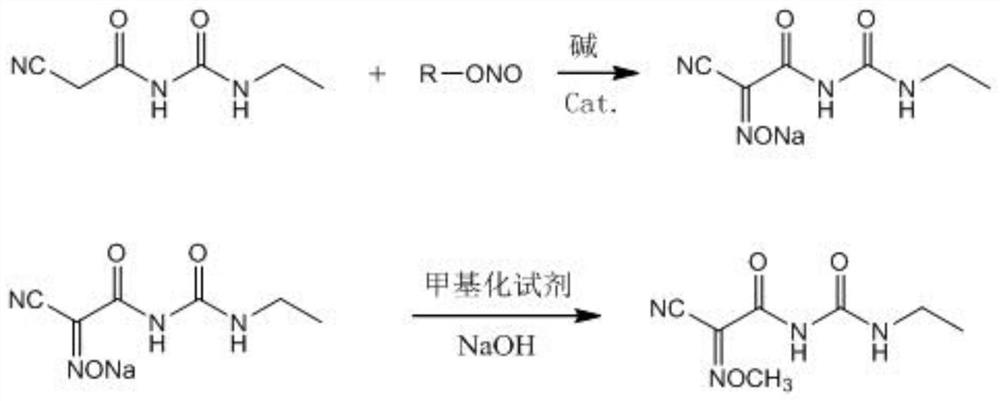
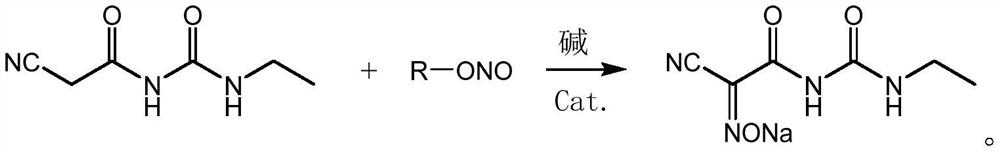
[0060] Add 86.2 g of cyanoacetoethyl urea (content 90%, 0.5 mol), 300 g of methanol, 27 g (0.5 mol) of sodium methoxide, and 0.087 g of DPhEG to a four-necked flask, stir to cool down to 0 °C, and slowly add isopropyl nitrite dropwise. 44.6 g (0.5 mol) of the ester was dripped, and the reaction was incubated for 8 h to obtain a light yellow turbid solution of 2-cyano-2-oximinoacetamide sodium salt.
[0061] The reaction solution in the previous step was heated to 30°C, and 45 g (0.5 mol) of dimethyl carbonate was added dropwise. The pH value was adjusted with liquid caustic soda in the range of 8 to 8.5 during the dropwise addition. Cool to -5°C for crystallization, filter, rinse the filter cake twice with water, and recover the solvent methanol by distillation of the mother liquor. The filter cake was dried to obtain 95.0 g of a white cyanamide product with a content of 99.1% and a yield of 95.0%.
Embodiment 2
[0063] In a four-necked flask, add 86.2 g of cyanoacetoethyl urea (content 90%, 0.5 mol), 300 g of ethanol, 37.4 g (0.55 mol) of sodium ethoxide, and 0.43 g of DPhEG, stir and cool down to 10 °C, and slowly add tertiary nitrite dropwise. 56.7 g (0.55 mol) of butyl ester was dripped, and the reaction was incubated for 6 h to obtain a light yellow turbid solution of 2-cyano-2-oximinoacetamide sodium salt.
[0064] The reaction solution in the previous step was heated to 40°C, dimethyl carbonate 49.5g (0.55mol) was added dropwise, the pH value was adjusted with liquid caustic soda in the range of 8 to 8.5 during the dropwise addition, the dropping was completed, the reaction was incubated for 3h, and the reaction was completed in HPLC. . Cool to 0°C for crystallization, filter, rinse the filter cake twice with water, and recover the solvent ethanol by distillation of the mother liquor. The filter cake was dried to obtain 95.4 g of a white cyanamide product with a content of 99.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com