Aromatic boron compound of conjugated olefin as well as preparation method and application of aromatic boron compound
A technology of conjugated olefins and compounds, which is applied in the field of aromatic boron compounds of conjugated olefins and its preparation, can solve the problems of difficult control of olefin regioselectivity, and achieve good regioselectivity, high yield, and mild reaction conditions Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] (1) Under an argon atmosphere, 0.2 mmol (about 57 mg) of conjugated olefin compound 2a, 0.6 mmol (about 152 mg) of pinacol biboronate, 0.01 mmol were sequentially added to a 25 mL Schlenk tube containing a magnetic stirring bar (about 3.7 mg) allylpalladium(II) chloride dimer, 0.02 mmol (about 9.4 mg) 2-dicyclohexylphosphine-2',6'-diisopropoxy-1,1'-bi Benzene, 0.6 mmol (about 82.9 mg) potassium carbonate, 0.3 mmol (about 32 uL) aryl bromide 1a (bromobenzene) were added to 2 mL of 1,4-dioxane, the tube was tightly capped, and the mixture was mixed In a preheated 100°C oil bath, vigorously stirring for 24 hours can complete the reaction;
[0082] (2) The reaction product was purified by silica gel column (triethylamine was used for silica gel, and PE:EA=2~20:1 as developing solvent) to obtain 77 mg of white conjugated olefin aryl boron compound 1 with a yield of 85 %.
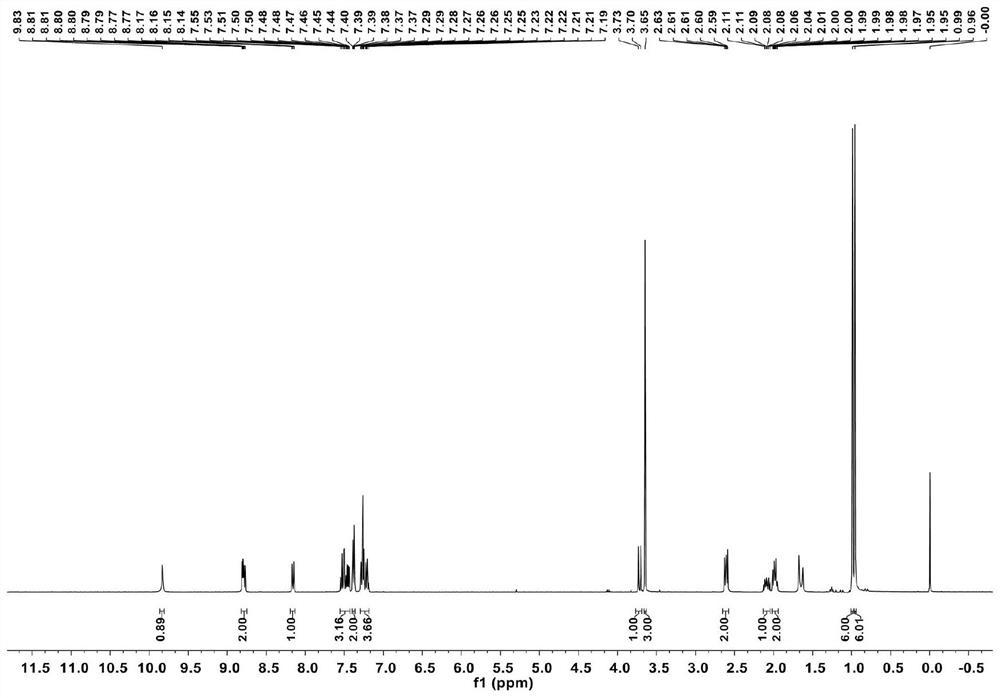
[0083] Characterize compound 1 as Figures 1 to 2 shown, figure 1 its 1 H NMR nuclear magnetic spe...
Embodiment 2
[0090] (1) Under an argon atmosphere, 0.2 mmol of conjugated olefin compound 2a, 0.4 mmol of pinacol biborate, and 0.002 mmol of allyl palladium chloride were sequentially added to a dry 25 mL Schlenk tube containing a magnetic stirring bar. (II) dimer, 0.001 mmol 2-dicyclohexylphosphorus-2'-methylbiphenyl, 0.3 mmol potassium carbonate, 0.2 mmol aryl bromide 1c (p-tert-butylbromobenzene) was added to 2 mL tert-amyl alcohol , the tube was tightly capped, and the mixture was vigorously stirred in a preheated 80°C oil bath for 18 hours to complete the reaction;
[0091] (2) Purify the reaction product through silica gel column (use triethylamine for silica gel, and use PE:EA=2~20:1 as developing solvent) to obtain 100 mg of white conjugated olefin aryl boron compound 2 with a yield of 92 %.
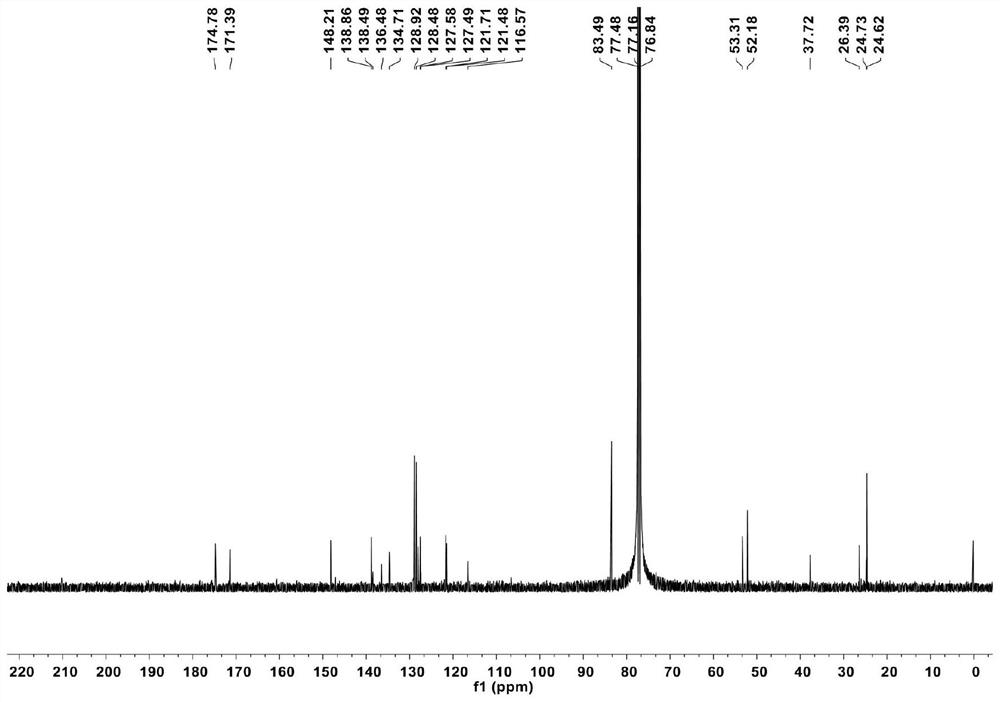
[0092] Characterize compound 2 as Figures 3 to 4 shown, where, image 3 its 1 H NMR nuclear magnetic spectrum, Figure 4 its 13 CNMR nuclear magnetic spectrum.
[0093] The character...
Embodiment 3
[0099] (1) Under an argon atmosphere, 0.2 mmol of conjugated olefin compound 2a, 1 mmol of biboronic acid pinacol ester, 0.02 mmol of allyl palladium chloride ( II) dimer, 0.04mmol 2-dicyclohexylphosphorus-2'-methylbiphenyl, 0.8mmol potassium carbonate, 0.6mmol aryl bromide 1f (p-bromotrifluoromethoxybenzene) was added to 2mL 1, In 4-dioxane, the tube was tightly capped, and the mixture was vigorously stirred in a preheated 120°C oil bath for 36 hours to complete the reaction;
[0100] (2) Purify the reaction product through silica gel column (use triethylamine for silica gel, and use PE:EA=2~20:1 as developing solvent) to obtain 65 mg of white conjugated olefin aryl boron compound 3 with a yield of 57 %.
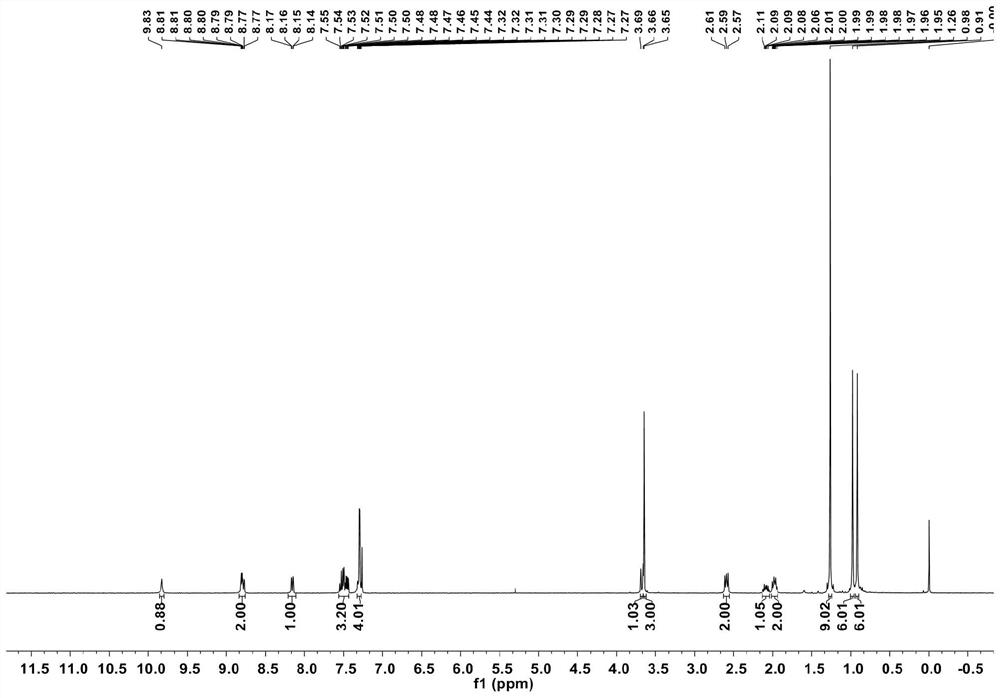
[0101] Compound 3 was characterized and the results were as follows Figures 5 to 7 shown, where, Figure 5 its 1 H NMR nuclear magnetic spectrum, Image 6 its 13 C NMR spectrum, Figure 7 its 19 F NMR nuclear magnetic spectrum.
[0102] The characterization data f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com